Vaginal Exposure to Zika Virus during Pregnancy Leads to Fetal Brain Infection
- PMID: 27565347
- PMCID: PMC5006689
- DOI: 10.1016/j.cell.2016.08.004
Vaginal Exposure to Zika Virus during Pregnancy Leads to Fetal Brain Infection
Abstract
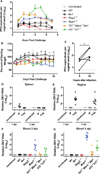
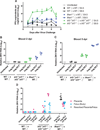
Zika virus (ZIKV) can be transmitted sexually between humans. However, it is unknown whether ZIKV replicates in the vagina and impacts the unborn fetus. Here, we establish a mouse model of vaginal ZIKV infection and demonstrate that, unlike other routes, ZIKV replicates within the genital mucosa even in wild-type (WT) mice. Mice lacking RNA sensors or transcription factors IRF3 and IRF7 resulted in higher levels of local viral replication. Furthermore, mice lacking the type I interferon (IFN) receptor (IFNAR) became viremic and died of infection after a high-dose vaginal ZIKV challenge. Notably, vaginal infection of pregnant dams during early pregnancy led to fetal growth restriction and infection of the fetal brain in WT mice. This was exacerbated in mice deficient in IFN pathways, leading to abortion. Our study highlights the vaginal tract as a highly susceptible site of ZIKV replication and illustrates the dire disease consequences during pregnancy.
Keywords: antiviral defense; female reproductive tract; flavivirus; innate immunity; mucosal immunity; pattern recognition receptors; sexually transmitted infections; type I interferons.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Type I interferons instigate fetal demise after Zika virus infection.Sci Immunol. 2018 Jan 5;3(19):eaao1680. doi: 10.1126/sciimmunol.aao1680. Sci Immunol. 2018. PMID: 29305462 Free PMC article.
-
Zika Virus Replicates in the Vagina of Mice with Intact Interferon Signaling.J Virol. 2022 Sep 28;96(18):e0121922. doi: 10.1128/jvi.01219-22. Epub 2022 Aug 30. J Virol. 2022. PMID: 36040178 Free PMC article.
-
Fetal Brain Infection Is Not a Unique Characteristic of Brazilian Zika Viruses.Viruses. 2018 Oct 3;10(10):541. doi: 10.3390/v10100541. Viruses. 2018. PMID: 30282919 Free PMC article.
-
Zika virus and reproduction: facts, questions and current management.Hum Reprod Update. 2017 Nov 1;23(6):629-645. doi: 10.1093/humupd/dmx024. Hum Reprod Update. 2017. PMID: 28961800 Review.
-
Immune Evasion Strategies Used by Zika Virus to Infect the Fetal Eye and Brain.Viral Immunol. 2020 Jan/Feb;33(1):22-37. doi: 10.1089/vim.2019.0082. Epub 2019 Nov 5. Viral Immunol. 2020. PMID: 31687902 Free PMC article. Review.
Cited by
-
Peli1 signaling blockade attenuates congenital zika syndrome.PLoS Pathog. 2020 Jun 16;16(6):e1008538. doi: 10.1371/journal.ppat.1008538. eCollection 2020 Jun. PLoS Pathog. 2020. PMID: 32544190 Free PMC article.
-
Role of microglia in brain development after viral infection.Front Cell Dev Biol. 2024 Jan 16;12:1340308. doi: 10.3389/fcell.2024.1340308. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38298216 Free PMC article. Review.
-
Inflammation-Induced, STING-Dependent Autophagy Restricts Zika Virus Infection in the Drosophila Brain.Cell Host Microbe. 2018 Jul 11;24(1):57-68.e3. doi: 10.1016/j.chom.2018.05.022. Epub 2018 Jun 19. Cell Host Microbe. 2018. PMID: 29934091 Free PMC article.
-
Type I interferons instigate fetal demise after Zika virus infection.Sci Immunol. 2018 Jan 5;3(19):eaao1680. doi: 10.1126/sciimmunol.aao1680. Sci Immunol. 2018. PMID: 29305462 Free PMC article.
-
Zika Virus Infects Trabecular Meshwork and Causes Trabeculitis and Glaucomatous Pathology in Mouse Eyes.mSphere. 2019 May 8;4(3):e00173-19. doi: 10.1128/mSphere.00173-19. mSphere. 2019. PMID: 31068433 Free PMC article.
References
-
- Bruni D, Chazal M, Sinigaglia L, Chauveau L, Schwartz O, Desprès P, Jouvenet N. Viral entry route determines how human plasmacytoid dendritic cells produce type I interferons. Sci. Signal. 2015;8 ra25. - PubMed
-
- Chambers TJ, Hahn CS, Galler R, Rice CM. Flavivirus genome organization, expression, and replication. Annu. Rev. Microbiol. 1990;44:649–688. - PubMed
-
- Crooks AJ, Lee JM, Easterbrook LM, Timofeev AV, Stephenson JR. The NS1 protein of tick-borne encephalitis virus forms multimeric species upon secretion from the host cell. J. Gen. Virol. 1994;75:3453–3460. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

