Identification and Optimization of the First Highly Selective GLUT1 Inhibitor BAY-876
- PMID: 27552707
- PMCID: PMC5095872
- DOI: 10.1002/cmdc.201600276
Identification and Optimization of the First Highly Selective GLUT1 Inhibitor BAY-876
Abstract
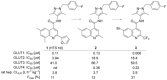
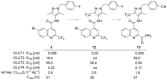
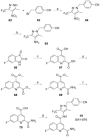
Despite the long-known fact that the facilitative glucose transporter GLUT1 is one of the key players safeguarding the increase in glucose consumption of many tumor entities even under conditions of normal oxygen supply (known as the Warburg effect), only few endeavors have been undertaken to find a GLUT1-selective small-molecule inhibitor. Because other transporters of the GLUT1 family are involved in crucial processes, these transporters should not be addressed by such an inhibitor. A high-throughput screen against a library of ∼3 million compounds was performed to find a small molecule with this challenging potency and selectivity profile. The N-(1H-pyrazol-4-yl)quinoline-4-carboxamides were identified as an excellent starting point for further compound optimization. After extensive structure-activity relationship explorations, single-digit nanomolar inhibitors with a selectivity factor of >100 against GLUT2, GLUT3, and GLUT4 were obtained. The most promising compound, BAY-876 [N4 -[1-(4-cyanobenzyl)-5-methyl-3-(trifluoromethyl)-1H-pyrazol-4-yl]-7-fluoroquinoline-2,4-dicarboxamide], showed good metabolic stability in vitro and high oral bioavailability in vivo.
Keywords: GLUT1 inhibitors; Warburg effect; medicinal chemistry; quinoline carboxamides; structure-activity relationships.
© 2016 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Figures
Similar articles
-
In Silico Modeling-based Identification of Glucose Transporter 4 (GLUT4)-selective Inhibitors for Cancer Therapy.J Biol Chem. 2015 Jun 5;290(23):14441-53. doi: 10.1074/jbc.M114.628826. Epub 2015 Apr 6. J Biol Chem. 2015. PMID: 25847249 Free PMC article.
-
Identification of novel GLUT inhibitors.Bioorg Med Chem Lett. 2016 Apr 1;26(7):1732-7. doi: 10.1016/j.bmcl.2016.02.050. Epub 2016 Feb 19. Bioorg Med Chem Lett. 2016. PMID: 26949183
-
Discovery of novel diarylpyrazolylquinoline derivatives as potent anti-dengue virus agents.Eur J Med Chem. 2017 Dec 1;141:282-292. doi: 10.1016/j.ejmech.2017.10.001. Epub 2017 Oct 3. Eur J Med Chem. 2017. PMID: 29031073
-
The progress and development of GLUT1 inhibitors targeting cancer energy metabolism.Future Med Chem. 2019 Sep;11(17):2333-2352. doi: 10.4155/fmc-2019-0052. Future Med Chem. 2019. PMID: 31581916 Review.
-
[The role of glucose transporter 1 (GLUT1) in the diagnosis and therapy of tumors].Postepy Hig Med Dosw (Online). 2012 Jan 4;66:165-74. doi: 10.5604/17322693.988242. Postepy Hig Med Dosw (Online). 2012. PMID: 22470192 Review. Polish.
Cited by
-
Dihydroceramide desaturase 1 (DES1) promotes anchorage-independent survival downstream of HER2-driven glucose uptake and metabolism.FASEB J. 2022 Oct;36(10):e22558. doi: 10.1096/fj.202200748R. FASEB J. 2022. PMID: 36165222 Free PMC article.
-
GLUT4 expression and glucose transport in human induced pluripotent stem cell-derived cardiomyocytes.PLoS One. 2019 Jul 25;14(7):e0217885. doi: 10.1371/journal.pone.0217885. eCollection 2019. PLoS One. 2019. PMID: 31344028 Free PMC article.
-
Erythroid glucose transport in health and disease.Pflugers Arch. 2020 Sep;472(9):1371-1383. doi: 10.1007/s00424-020-02406-0. Epub 2020 May 30. Pflugers Arch. 2020. PMID: 32474749 Review.
-
Different Glucose Metabolic Features According to Cancer and Immune Cells in the Tumor Microenvironment.Front Oncol. 2021 Dec 13;11:769393. doi: 10.3389/fonc.2021.769393. eCollection 2021. Front Oncol. 2021. PMID: 34966676 Free PMC article.
-
Endocytic vesicles act as vehicles for glucose uptake in response to growth factor stimulation.Nat Commun. 2024 Apr 2;15(1):2843. doi: 10.1038/s41467-024-46971-9. Nat Commun. 2024. PMID: 38565573 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

