Therapeutic Inhibition of the MDM2-p53 Interaction Prevents Recurrence of Adenoid Cystic Carcinomas
- PMID: 27550999
- PMCID: PMC5315632
- DOI: 10.1158/1078-0432.CCR-16-1235
Therapeutic Inhibition of the MDM2-p53 Interaction Prevents Recurrence of Adenoid Cystic Carcinomas
Abstract
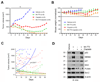
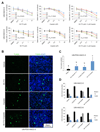
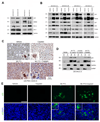
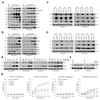
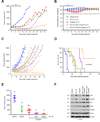
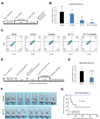
Purpose: Conventional chemotherapy has modest efficacy in advanced adenoid cystic carcinomas (ACC). Tumor recurrence is a major challenge in the management of ACC patients. Here, we evaluated the antitumor effect of a novel small-molecule inhibitor of the MDM2-p53 interaction (MI-773) combined with cisplatin in patient-derived xenograft (PDX) ACC tumors.Experimental Design: Therapeutic strategies with MI-773 and/or cisplatin were evaluated in SCID mice harboring PDX ACC tumors (UM-PDX-HACC-5) and in low passage primary human ACC cells (UM-HACC-2A, -2B, -5, -6) in vitro The effect of therapy on the fraction of cancer stem cells (CSC) was determined by flow cytometry for ALDH activity and CD44 expression.Results: Combined therapy with MI-773 with cisplatin caused p53 activation, induction of apoptosis, and regression of ACC PDX tumors. Western blots revealed induction of MDM2, p53 and downstream p21 expression, and regulation of apoptosis-related proteins PUMA, BAX, Bcl-2, Bcl-xL, and active caspase-9 upon MI-773 treatment. Both single-agent MI-773 and MI-773 combined with cisplatin decreased the fraction of CSCs in PDX ACC tumors. Notably, neoadjuvant MI-773 and surgery eliminated tumor recurrences during a postsurgical follow-up of more than 300 days. In contrast, 62.5% of mice that received vehicle control presented with palpable tumor recurrences within this time period (P = 0.0097).Conclusions: Collectively, these data demonstrate that therapeutic inhibition of MDM2-p53 interaction by MI-773 decreased the CSC fraction, sensitized ACC xenograft tumors to cisplatin, and eliminated tumor recurrence. These results suggest that patients with ACC might benefit from the therapeutic inhibition of the MDM2-p53 interaction. Clin Cancer Res; 23(4); 1036-48. ©2016 AACR.
©2016 American Association for Cancer Research.
Figures
Similar articles
-
Targeting MDM2 for Treatment of Adenoid Cystic Carcinoma.Clin Cancer Res. 2016 Jul 15;22(14):3550-9. doi: 10.1158/1078-0432.CCR-15-1698. Epub 2016 Mar 2. Clin Cancer Res. 2016. PMID: 26936915 Free PMC article.
-
Ablation of Cancer Stem Cells by Therapeutic Inhibition of the MDM2-p53 Interaction in Mucoepidermoid Carcinoma.Clin Cancer Res. 2019 Mar 1;25(5):1588-1600. doi: 10.1158/1078-0432.CCR-17-2730. Epub 2018 Nov 29. Clin Cancer Res. 2019. PMID: 30498096 Free PMC article.
-
Therapeutic inhibition of Bmi-1 ablates chemoresistant cancer stem cells in adenoid cystic carcinoma.Oral Oncol. 2023 Jul;142:106437. doi: 10.1016/j.oraloncology.2023.106437. Epub 2023 May 31. Oral Oncol. 2023. PMID: 37267716 Free PMC article.
-
Treatment of Recurrent or Metastatic Adenoid Cystic Carcinoma.Curr Oncol Rep. 2022 May;24(5):621-631. doi: 10.1007/s11912-022-01233-z. Epub 2022 Feb 25. Curr Oncol Rep. 2022. PMID: 35212920 Review.
-
Therapeutic potential of p53 reactivation in cervical cancer.Crit Rev Oncol Hematol. 2021 Jan;157:103182. doi: 10.1016/j.critrevonc.2020.103182. Epub 2020 Nov 24. Crit Rev Oncol Hematol. 2021. PMID: 33276182 Review.
Cited by
-
MDM2 Inhibitors for Cancer Therapy: The Past, Present, and Future.Pharmacol Rev. 2024 May 2;76(3):414-453. doi: 10.1124/pharmrev.123.001026. Pharmacol Rev. 2024. PMID: 38697854 Free PMC article. Review.
-
Molecular Aspects of Mucoepidermoid Carcinoma and Adenoid Cystic Carcinoma of the Salivary Gland.Head Neck Pathol. 2024 Apr 24;18(1):34. doi: 10.1007/s12105-024-01629-2. Head Neck Pathol. 2024. PMID: 38658430 Review.
-
The role of E3 ubiquitin ligase HECTD3 in cancer and beyond.Cell Mol Life Sci. 2020 Apr;77(8):1483-1495. doi: 10.1007/s00018-019-03339-3. Epub 2019 Oct 21. Cell Mol Life Sci. 2020. PMID: 31637449 Free PMC article. Review.
-
Bmi-1: A master regulator of head and neck cancer stemness.Front Oral Health. 2023 Jan 16;4:1080255. doi: 10.3389/froh.2023.1080255. eCollection 2023. Front Oral Health. 2023. PMID: 36726797 Free PMC article. Review.
-
mTOR Inhibition Ablates Cisplatin-Resistant Salivary Gland Cancer Stem Cells.J Dent Res. 2021 Apr;100(4):377-386. doi: 10.1177/0022034520965141. Epub 2020 Oct 17. J Dent Res. 2021. PMID: 33073679 Free PMC article.
References
-
- Coca-Pelaz A, Rodrigo JP, Bradley PJ, Vander Poorten V, Triantafyllou A, Hunt JL, et al. Adenoid Cystic carcinoma of the head and neck – An update. Oral Oncol. 2015;51:652–661. - PubMed
-
- Kowalski PJ, Paulino AF. Perineural invasion in adenoid cystic carcinoma: Its causation/promotion by brain-derived neurotrophic factor. Hum Pathol. 2002;33:933–936. - PubMed
-
- Dodd RL, Slevin NJ. Salivary gland adenoid cystic carcinoma: a review of chemotherapy and molecular therapies. Oral Oncol. 2006;42:759–769. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

