β-arrestin-2 regulates NMDA receptor function in spinal lamina II neurons and duration of persistent pain
- PMID: 27538456
- PMCID: PMC5477285
- DOI: 10.1038/ncomms12531
β-arrestin-2 regulates NMDA receptor function in spinal lamina II neurons and duration of persistent pain
Abstract
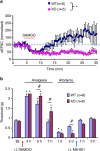
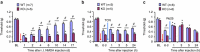
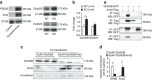
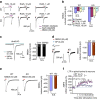
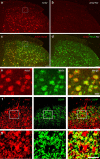
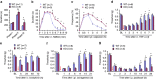
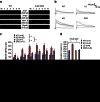
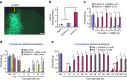
Mechanisms of acute pain transition to chronic pain are not fully understood. Here we demonstrate an active role of β-arrestin 2 (Arrb2) in regulating spinal cord NMDA receptor (NMDAR) function and the duration of pain. Intrathecal injection of the mu-opioid receptor agonist [D-Ala(2), NMe-Phe(4), Gly-ol(5)]-enkephalin produces paradoxical behavioural responses: early-phase analgesia and late-phase mechanical allodynia which requires NMDAR; both phases are prolonged in Arrb2 knockout (KO) mice. Spinal administration of NMDA induces GluN2B-dependent mechanical allodynia, which is prolonged in Arrb2-KO mice and conditional KO mice lacking Arrb2 in presynaptic terminals expressing Nav1.8. Loss of Arrb2 also results in prolongation of inflammatory pain and neuropathic pain and enhancement of GluN2B-mediated NMDA currents in spinal lamina IIo not lamina I neurons. Finally, spinal over-expression of Arrb2 reverses chronic neuropathic pain after nerve injury. Thus, spinal Arrb2 may serve as an intracellular gate for acute to chronic pain transition via desensitization of NMDAR.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Similar articles
-
Intrathecal clonidine suppresses phosphorylation of the N-methyl-D-aspartate receptor NR1 subunit in spinal dorsal horn neurons of rats with neuropathic pain.Anesth Analg. 2008 Aug;107(2):693-700. doi: 10.1213/ane.0b013e31817e7319. Anesth Analg. 2008. PMID: 18633054
-
Involvement of Brain-Enriched Guanylate Kinase-Associated Protein (BEGAIN) in Chronic Pain after Peripheral Nerve Injury.eNeuro. 2016 Oct 17;3(5):ENEURO.0110-16.2016. doi: 10.1523/ENEURO.0110-16.2016. eCollection 2016 Sep-Oct. eNeuro. 2016. PMID: 27785460 Free PMC article.
-
Activation of spinal alpha-2 adrenoceptors, but not mu-opioid receptors, reduces the intrathecal N-methyl-D-aspartate-induced increase in spinal NR1 subunit phosphorylation and nociceptive behaviors in the rat.Anesth Analg. 2010 Feb 1;110(2):622-9. doi: 10.1213/ANE.0b013e3181c8afc1. Epub 2009 Dec 10. Anesth Analg. 2010. PMID: 20007733
-
[Involvement of spinally-infiltrated immune cells in peripheral nerve injury-induced neuropathic pain: roles of TRPM2].Nihon Yakurigaku Zasshi. 2013 Nov;142(5):215-20. doi: 10.1254/fpj.142.215. Nihon Yakurigaku Zasshi. 2013. PMID: 24212589 Review. Japanese. No abstract available.
-
The role of the extracellular matrix in chronic pain following injury.Pain. 2015 Mar;156(3):366-370. doi: 10.1097/01.j.pain.0000460323.80020.9d. Pain. 2015. PMID: 25679468 Review. No abstract available.
Cited by
-
Rubbing Salt in the Wound: Molecular Evolutionary Analysis of Pain-Related Genes Reveals the Pain Adaptation of Cetaceans in Seawater.Animals (Basel). 2022 Dec 16;12(24):3571. doi: 10.3390/ani12243571. Animals (Basel). 2022. PMID: 36552490 Free PMC article.
-
Sex-Dependent Glial Signaling in Pathological Pain: Distinct Roles of Spinal Microglia and Astrocytes.Neurosci Bull. 2018 Feb;34(1):98-108. doi: 10.1007/s12264-017-0145-y. Epub 2017 Jun 5. Neurosci Bull. 2018. PMID: 28585113 Free PMC article.
-
CCL2 facilitates spinal synaptic transmission and pain via interaction with presynaptic CCR2 in spinal nociceptor terminals.Mol Brain. 2020 Nov 23;13(1):161. doi: 10.1186/s13041-020-00701-6. Mol Brain. 2020. PMID: 33228784 Free PMC article.
-
Activated spinal astrocytes contribute to the later phase of carrageenan-induced prostatitis pain.J Neuroinflammation. 2019 Oct 25;16(1):189. doi: 10.1186/s12974-019-1584-3. J Neuroinflammation. 2019. PMID: 31653262 Free PMC article.
-
The Involvement of Caspases in Neuroinflammation and Neuronal Apoptosis in Chronic Pain and Potential Therapeutic Targets.Front Pharmacol. 2022 May 3;13:898574. doi: 10.3389/fphar.2022.898574. eCollection 2022. Front Pharmacol. 2022. PMID: 35592413 Free PMC article. Review.
References
-
- Hucho T. & Levine J. D. Signaling pathways in sensitization: toward a nociceptor cell biology. Neuron 55, 365–376 (2007). - PubMed
-
- Woolf C. J. & Salter M. W. Neuronal plasticity: increasing the gain in pain. Science 288, 1765–1769 (2000). - PubMed
-
- Ji R. R., Kohno T., Moore K. A. & Woolf C. J. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci. 26, 696–705 (2003). - PubMed
-
- Kuner R. Central mechanisms of pathological pain. Nat. Med. 16, 1258–1266 (2010). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

