The Pseudokinase MLKL and the Kinase RIPK3 Have Distinct Roles in Autoimmune Disease Caused by Loss of Death-Receptor-Induced Apoptosis
- PMID: 27523270
- PMCID: PMC5040700
- DOI: 10.1016/j.immuni.2016.07.016
The Pseudokinase MLKL and the Kinase RIPK3 Have Distinct Roles in Autoimmune Disease Caused by Loss of Death-Receptor-Induced Apoptosis
Abstract
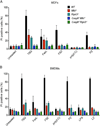
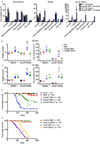
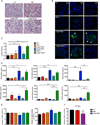
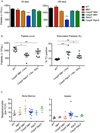
The kinases RIPK1 and RIPK3 and the pseudo-kinase MLKL have been identified as key regulators of the necroptotic cell death pathway, although a role for MLKL within the whole animal has not yet been established. Here, we have shown that MLKL deficiency rescued the embryonic lethality caused by loss of Caspase-8 or FADD. Casp8(-/-)Mlkl(-/-) and Fadd(-/-)Mlkl(-/-) mice were viable and fertile but rapidly developed severe lymphadenopathy, systemic autoimmune disease, and thrombocytopenia. These morbidities occurred more rapidly and with increased severity in Casp8(-/-)Mlkl(-/-) and Fadd(-/-)Mlkl(-/-) mice compared to Casp8(-/-)Ripk3(-/-) or Fadd(-/-)Ripk3(-/-) mice, respectively. These results demonstrate that MLKL is an essential effector of aberrant necroptosis in embryos caused by loss of Caspase-8 or FADD. Furthermore, they suggest that RIPK3 and/or MLKL may exert functions independently of necroptosis. It appears that non-necroptotic functions of RIPK3 contribute to the lymphadenopathy, autoimmunity, and excess cytokine production that occur when FADD or Caspase-8-mediated apoptosis is abrogated.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Caspase-8 auto-cleavage regulates programmed cell death and collaborates with RIPK3/MLKL to prevent lymphopenia.Cell Death Differ. 2022 Aug;29(8):1500-1512. doi: 10.1038/s41418-022-00938-9. Epub 2022 Jan 21. Cell Death Differ. 2022. PMID: 35064213 Free PMC article.
-
The neurotoxicant PCB-95 by increasing the neuronal transcriptional repressor REST down-regulates caspase-8 and increases Ripk1, Ripk3 and MLKL expression determining necroptotic neuronal death.Biochem Pharmacol. 2017 Oct 15;142:229-241. doi: 10.1016/j.bcp.2017.06.135. Epub 2017 Jul 1. Biochem Pharmacol. 2017. PMID: 28676433
-
Caspase-8 and FADD prevent spontaneous ZBP1 expression and necroptosis.Proc Natl Acad Sci U S A. 2022 Oct 11;119(41):e2207240119. doi: 10.1073/pnas.2207240119. Epub 2022 Oct 3. Proc Natl Acad Sci U S A. 2022. PMID: 36191211 Free PMC article.
-
Necroptosis in development, inflammation and disease.Nat Rev Mol Cell Biol. 2017 Feb;18(2):127-136. doi: 10.1038/nrm.2016.149. Epub 2016 Dec 21. Nat Rev Mol Cell Biol. 2017. PMID: 27999438 Review.
-
Necroptotic Cell Death Signaling and Execution Pathway: Lessons from Knockout Mice.Mediators Inflamm. 2015;2015:128076. doi: 10.1155/2015/128076. Epub 2015 Sep 27. Mediators Inflamm. 2015. PMID: 26491219 Free PMC article. Review.
Cited by
-
Hyperlipidemia-induced hematopoiesis is repressed by MLKL in endothelial cells of the splenic niche.Nat Cardiovasc Res. 2024 May;3(5):594-611. doi: 10.1038/s44161-024-00470-8. Epub 2024 May 17. Nat Cardiovasc Res. 2024. PMID: 39195940
-
Caspase-8 auto-cleavage regulates programmed cell death and collaborates with RIPK3/MLKL to prevent lymphopenia.Cell Death Differ. 2022 Aug;29(8):1500-1512. doi: 10.1038/s41418-022-00938-9. Epub 2022 Jan 21. Cell Death Differ. 2022. PMID: 35064213 Free PMC article.
-
The p55TNFR-IKK2-Ripk3 axis orchestrates arthritis by regulating death and inflammatory pathways in synovial fibroblasts.Nat Commun. 2018 Feb 12;9(1):618. doi: 10.1038/s41467-018-02935-4. Nat Commun. 2018. PMID: 29434332 Free PMC article.
-
The 'cytokine storm': molecular mechanisms and therapeutic prospects.Trends Immunol. 2021 Aug;42(8):681-705. doi: 10.1016/j.it.2021.06.001. Epub 2021 Jul 1. Trends Immunol. 2021. PMID: 34217595 Free PMC article. Review.
-
Induction of necroptotic cell death by viral activation of the RIG-I or STING pathway.Cell Death Differ. 2017 Apr;24(4):615-625. doi: 10.1038/cdd.2016.153. Epub 2017 Jan 6. Cell Death Differ. 2017. PMID: 28060376 Free PMC article.
References
-
- Bossen C, Ingold K, Tardivel A, Bodmer JL, Gaide O, Hertig S, Ambrose C, Tschopp J, Schneider P. Interactions of tumor necrosis factor (TNF) and TNF receptor family members in the mouse and human. The Journal of biological chemistry. 2006;281:13964–13971. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

