Early oral immunotherapy in peanut-allergic preschool children is safe and highly effective
- PMID: 27522159
- PMCID: PMC5222765
- DOI: 10.1016/j.jaci.2016.05.027
Early oral immunotherapy in peanut-allergic preschool children is safe and highly effective
Abstract
Background: Oral immunotherapy (OIT) is an effective experimental food allergy treatment that is limited by treatment withdrawal and the frequent reversibility of desensitization if interrupted. Newly diagnosed preschool children may have clinical and immunological characteristics more amenable to treatment.
Objective: We sought to test the safety, effectiveness, and feasibility of early OIT (E-OIT) in the treatment of peanut allergy.
Methods: We enrolled 40 children aged 9 to 36 months with suspected or known peanut allergy. Qualifying subjects reacted to peanut during an entry food challenge and were block-randomized 1:1 to receive E-OIT at goal maintenance doses of 300 or 3000 mg/d in a double-blinded fashion. The primary end point, sustained unresponsiveness at 4 weeks after stopping early intervention oral immunotherapy (4-SU), was assessed by double-blinded, placebo-controlled food challenge either upon achieving 4 prespecified criteria, or after 3 maintenance years. Peanut-specific immune responses were serially analyzed. Outcomes were compared with 154 matched standard-care controls.
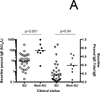
Results: Of 40 consented subjects, 3 (7.5%) did not qualify. Overall, 29 of 37 (78%) in the intent-to-treat analysis achieved 4-SU (300-mg arm, 17 of 20 [85%]; 3000 mg, 12 of 17 [71%], P = .43) over a median of 29 months. Per-protocol, the overall proportion achieving 4-SU was 29 of 32 (91%). Peanut-specific IgE levels significantly declined in E-OIT-treated children, who were 19 times more likely to successfully consume dietary peanut than matched standard-care controls, in whom peanut-specific IgE levels significantly increased (relative risk, 19.42; 95% CI, 8.7-43.7; P < .001). Allergic side effects during E-OIT were common but all were mild to moderate.
Conclusions: At both doses tested, E-OIT had an acceptable safety profile and was highly successful in rapidly suppressing allergic immune responses and achieving safe dietary reintroduction.
Keywords: Oral immunotherapy; desensitization; early intervention; peanut allergy; randomized clinical trial; sustained unresponsiveness.
Copyright © 2016 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Figures









Comment in
-
Recent advances in food allergy prevention and treatment.Ann Allergy Asthma Immunol. 2018 Mar;120(3):241-244. doi: 10.1016/j.anai.2018.01.023. Epub 2018 Feb 2. Ann Allergy Asthma Immunol. 2018. PMID: 29409852 No abstract available.
Similar articles
-
Sustained unresponsiveness to peanut in subjects who have completed peanut oral immunotherapy.J Allergy Clin Immunol. 2014 Feb;133(2):468-75. doi: 10.1016/j.jaci.2013.11.007. Epub 2013 Dec 19. J Allergy Clin Immunol. 2014. PMID: 24361082 Free PMC article. Clinical Trial.
-
Administration of a probiotic with peanut oral immunotherapy: A randomized trial.J Allergy Clin Immunol. 2015 Mar;135(3):737-44.e8. doi: 10.1016/j.jaci.2014.11.034. Epub 2015 Jan 13. J Allergy Clin Immunol. 2015. PMID: 25592987 Clinical Trial.
-
Probiotic peanut oral immunotherapy versus oral immunotherapy and placebo in children with peanut allergy in Australia (PPOIT-003): a multicentre, randomised, phase 2b trial.Lancet Child Adolesc Health. 2022 Mar;6(3):171-184. doi: 10.1016/S2352-4642(22)00006-2. Epub 2022 Feb 4. Lancet Child Adolesc Health. 2022. PMID: 35123664 Clinical Trial.
-
Allergen-specific oral immunotherapy for peanut allergy.Cochrane Database Syst Rev. 2012 Sep 12;2012(9):CD009014. doi: 10.1002/14651858.CD009014.pub2. Cochrane Database Syst Rev. 2012. PMID: 22972130 Free PMC article. Review.
-
Peanut Oral Immunotherapy: a Current Perspective.Curr Allergy Asthma Rep. 2020 Apr 20;20(5):14. doi: 10.1007/s11882-020-00908-6. Curr Allergy Asthma Rep. 2020. PMID: 32314071 Free PMC article. Review.
Cited by
-
A Practical, Stepwise Approach to Peanut Oral Immunotherapy in Clinical Practice: Benefits and Risks.J Asthma Allergy. 2021 Mar 25;14:277-285. doi: 10.2147/JAA.S290915. eCollection 2021. J Asthma Allergy. 2021. PMID: 33790583 Free PMC article. Review.
-
Oral immunotherapy for multiple foods in a pediatric allergy clinic setting.Ann Allergy Asthma Immunol. 2019 Dec;123(6):573-581.e3. doi: 10.1016/j.anai.2019.08.463. Epub 2019 Sep 6. Ann Allergy Asthma Immunol. 2019. PMID: 31494236 Free PMC article.
-
Evolution of Immune Responses in Food Immunotherapy.Immunol Allergy Clin North Am. 2020 Feb;40(1):87-95. doi: 10.1016/j.iac.2019.09.006. Epub 2019 Nov 6. Immunol Allergy Clin North Am. 2020. PMID: 31761123 Free PMC article. Review.
-
Current Controversies and Future Prospects for Peanut Allergy Prevention, Diagnosis and Therapies.J Asthma Allergy. 2020 Jan 16;13:51-66. doi: 10.2147/JAA.S196268. eCollection 2020. J Asthma Allergy. 2020. PMID: 32021312 Free PMC article. Review.
-
Eosinophilic esophagitis during sublingual and oral allergen immunotherapy.Curr Opin Allergy Clin Immunol. 2019 Aug;19(4):350-357. doi: 10.1097/ACI.0000000000000537. Curr Opin Allergy Clin Immunol. 2019. PMID: 31058677 Free PMC article. Review.
References
-
- Sicherer SH, Munoz-Furlong A, Godbold JH, Sampson HA. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. The Journal of allergy and clinical immunology. 2010;125(6):1322–1326. - PubMed
-
- Osborne NJ, Koplin JJ, Martin PE, Gurrin LC, Lowe AJ, Matheson MC, et al. Prevalence of challenge-proven IgE-mediated food allergy using population-based sampling and predetermined challenge criteria in infants. The Journal of allergy and clinical immunology. 2011;127(3):668–676. e1–e2. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

