Exosome-mediated Delivery of Hydrophobically Modified siRNA for Huntingtin mRNA Silencing
- PMID: 27506293
- PMCID: PMC5112038
- DOI: 10.1038/mt.2016.126
Exosome-mediated Delivery of Hydrophobically Modified siRNA for Huntingtin mRNA Silencing
Abstract
Delivery represents a significant barrier to the clinical advancement of oligonucleotide therapeutics for the treatment of neurological disorders, such as Huntington's disease. Small, endogenous vesicles known as exosomes have the potential to act as oligonucleotide delivery vehicles, but robust and scalable methods for loading RNA therapeutic cargo into exosomes are lacking. Here, we show that hydrophobically modified small interfering RNAs (hsiRNAs) efficiently load into exosomes upon co-incubation, without altering vesicle size distribution or integrity. Exosomes loaded with hsiRNAs targeting Huntingtin mRNA were efficiently internalized by mouse primary cortical neurons and promoted dose-dependent silencing of Huntingtin mRNA and protein. Unilateral infusion of hsiRNA-loaded exosomes, but not hsiRNAs alone, into mouse striatum resulted in bilateral oligonucleotide distribution and statistically significant bilateral silencing of up to 35% of Huntingtin mRNA. The broad distribution and efficacy of hsiRNA-loaded exosomes delivered to brain is expected to advance the development of therapies for the treatment of Huntington's disease and other neurodegenerative disorders.
Figures
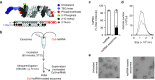
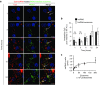
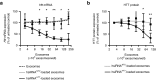
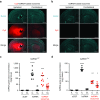

Similar articles
-
Loading of Extracellular Vesicles with Hydrophobically Modified siRNAs.Methods Mol Biol. 2018;1740:199-214. doi: 10.1007/978-1-4939-7652-2_16. Methods Mol Biol. 2018. PMID: 29388146
-
Transvascular Delivery of Hydrophobically Modified siRNAs: Gene Silencing in the Rat Brain upon Disruption of the Blood-Brain Barrier.Mol Ther. 2018 Nov 7;26(11):2580-2591. doi: 10.1016/j.ymthe.2018.08.005. Epub 2018 Aug 8. Mol Ther. 2018. PMID: 30143435 Free PMC article.
-
Optimized Cholesterol-siRNA Chemistry Improves Productive Loading onto Extracellular Vesicles.Mol Ther. 2018 Aug 1;26(8):1973-1982. doi: 10.1016/j.ymthe.2018.05.024. Epub 2018 Jun 21. Mol Ther. 2018. PMID: 29937418 Free PMC article.
-
Emerging Roles of Exosomes in Huntington's Disease.Int J Mol Sci. 2021 Apr 15;22(8):4085. doi: 10.3390/ijms22084085. Int J Mol Sci. 2021. PMID: 33920936 Free PMC article. Review.
-
Nucleic Acid Therapeutics in Huntington's Disease.Recent Pat Biotechnol. 2019;13(3):187-206. doi: 10.2174/1872208313666190208163714. Recent Pat Biotechnol. 2019. PMID: 30747088 Review.
Cited by
-
Combinatorial microRNA Loading into Extracellular Vesicles for Increased Anti-Inflammatory Efficacy.Noncoding RNA. 2022 Oct 21;8(5):71. doi: 10.3390/ncrna8050071. Noncoding RNA. 2022. PMID: 36287123 Free PMC article.
-
Exosome-derived CIRP: An amplifier of inflammatory diseases.Front Immunol. 2023 Feb 14;14:1066721. doi: 10.3389/fimmu.2023.1066721. eCollection 2023. Front Immunol. 2023. PMID: 36865547 Free PMC article. Review.
-
Rapid On-Demand Extracellular Vesicle Augmentation with Versatile Oligonucleotide Tethers.ACS Nano. 2019 Sep 24;13(9):10555-10565. doi: 10.1021/acsnano.9b04651. Epub 2019 Aug 27. ACS Nano. 2019. PMID: 31436946 Free PMC article.
-
Intracellular and intercellular transport of RNA organelles in CXG repeat disorders: The strength of weak ties.Front Mol Biosci. 2022 Dec 16;9:1000932. doi: 10.3389/fmolb.2022.1000932. eCollection 2022. Front Mol Biosci. 2022. PMID: 36589236 Free PMC article. Review.
-
In vitro experimental study on the formation of microRNA-34a loaded exosomes and their inhibitory effect in oral squamous cell carcinoma.Cell Cycle. 2022 Aug;21(16):1775-1783. doi: 10.1080/15384101.2022.2070832. Epub 2022 Apr 29. Cell Cycle. 2022. PMID: 35485349 Free PMC article.
References
-
- HD Consortium (1993). A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell 72: 971–983. - PubMed
-
- Ross, CA and Tabrizi, SJ (2011). Huntington's disease: from molecular pathogenesis to clinical treatment. Lancet Neurol 10: 83–98. - PubMed
-
- Warby, SC, Graham, RK and Hayden, MR (2010). Huntington Disease. GeneReviewsTM. <http://www.ncbi.nlm.nih.gov/books/NBK1305/>.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

