Marked Sexual Dimorphism in the Role of the Ryanodine Receptor in a Model of Pain Chronification in the Rat
- PMID: 27499186
- PMCID: PMC4976309
- DOI: 10.1038/srep31221
Marked Sexual Dimorphism in the Role of the Ryanodine Receptor in a Model of Pain Chronification in the Rat
Abstract
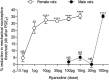
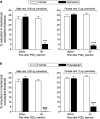
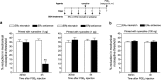
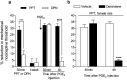
Hyperalgesic priming, an estrogen dependent model of the transition to chronic pain, produced by agonists at receptors that activate protein kinase C epsilon (PKCε), occurs in male but not in female rats. However, activation of second messengers downstream of PKCε, such as the ryanodine receptor, induces priming in both sexes. Since estrogen regulates intracellular calcium, we investigated the interaction between estrogen and ryanodine in the susceptibility to develop priming in females. The lowest dose of ryanodine able to induce priming in females (1 pg) is 1/100,000(th) that needed in males (100 ng), an effect dependent on the activation of ryanodine receptors. Treatment of female rats with antisense to estrogen receptor alpha (ERα), but not beta (ERβ), mRNA, prevented the induction of priming by low dose ryanodine, and the ERα agonist, PPT, induced ryanodine receptor-dependent priming. In vitro application of ryanodine in low concentration (2 nM) to small DRG neurons cultured from females, significantly potentiated calcium release via ryanodine receptors induced by caffeine. This effect was only observed in IB4+ neurons, cultured in the presence of β-estradiol or PPT. Our results demonstrate a profound regulatory role of ERα in ryanodine receptor-dependent transition to chronic pain.
Figures

Similar articles
-
Sexual Dimorphism in a Reciprocal Interaction of Ryanodine and IP3 Receptors in the Induction of Hyperalgesic Priming.J Neurosci. 2017 Feb 22;37(8):2032-2044. doi: 10.1523/JNEUROSCI.2911-16.2017. Epub 2017 Jan 23. J Neurosci. 2017. PMID: 28115480 Free PMC article.
-
Second messengers mediating the expression of neuroplasticity in a model of chronic pain in the rat.J Pain. 2014 Mar;15(3):312-20. doi: 10.1016/j.jpain.2013.12.005. Epub 2014 Jan 7. J Pain. 2014. PMID: 24407022 Free PMC article.
-
Regulation of Expression of Hyperalgesic Priming by Estrogen Receptor α in the Rat.J Pain. 2017 May;18(5):574-582. doi: 10.1016/j.jpain.2016.12.017. Epub 2017 Jan 9. J Pain. 2017. PMID: 28089711 Free PMC article.
-
Role of nociceptor αCaMKII in transition from acute to chronic pain (hyperalgesic priming) in male and female rats.J Neurosci. 2013 Jul 3;33(27):11002-11. doi: 10.1523/JNEUROSCI.1785-13.2013. J Neurosci. 2013. PMID: 23825405 Free PMC article.
-
Neuregulin beta1 enhances peak glutamate-induced intracellular calcium levels through endoplasmic reticulum calcium release in cultured hippocampal neurons.Can J Physiol Pharmacol. 2009 Oct;87(10):883-91. doi: 10.1139/Y09-082. Can J Physiol Pharmacol. 2009. PMID: 20052014
Cited by
-
Mechanisms Mediating High-Molecular-Weight Hyaluronan-Induced Antihyperalgesia.J Neurosci. 2020 Aug 19;40(34):6477-6488. doi: 10.1523/JNEUROSCI.0166-20.2020. Epub 2020 Jul 14. J Neurosci. 2020. PMID: 32665406 Free PMC article.
-
In Vitro Nociceptor Neuroplasticity Associated with In Vivo Opioid-Induced Hyperalgesia.J Neurosci. 2019 Sep 4;39(36):7061-7073. doi: 10.1523/JNEUROSCI.1191-19.2019. Epub 2019 Jul 12. J Neurosci. 2019. PMID: 31300521 Free PMC article.
-
Sexual dimorphism in the nociceptive effects of hyaluronan.Pain. 2021 Apr 1;162(4):1116-1125. doi: 10.1097/j.pain.0000000000002116. Pain. 2021. PMID: 33065736 Free PMC article.
-
Endoplasmic reticulum-mitochondria interplay in chronic pain: The calcium connection.Mol Pain. 2020 Jan-Dec;16:1744806920946889. doi: 10.1177/1744806920946889. Mol Pain. 2020. PMID: 32787562 Free PMC article. Review.
-
Systemic Morphine Produces Dose-dependent Nociceptor-mediated Biphasic Changes in Nociceptive Threshold and Neuroplasticity.Neuroscience. 2019 Feb 1;398:64-75. doi: 10.1016/j.neuroscience.2018.11.051. Epub 2018 Dec 7. Neuroscience. 2019. PMID: 30529265 Free PMC article.
References
-
- Buse D. C. et al.. Sex differences in the prevalence, symptoms, and associated features of migraine, probable migraine and other severe headache: results of the American Migraine Prevalence and Prevention (AMPP) Study. Headache 53, 1278–1299 (2013). - PubMed
-
- de Mos M., Huygen F. J., Stricker B. H., Dieleman J. P. & Sturkenboom M. C. Estrogens and the risk of complex regional pain syndrome (CRPS). Pharmacoepidemiol Drug Saf 18, 44–52 (2009). - PubMed
-
- Ouyang A. & Wrzos H. F. Contribution of gender to pathophysiology and clinical presentation of IBS: should management be different in women? Am J Gastroenterol 101, S602–9 (2006). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

