Plant-Mediated Effects on Mosquito Capacity to Transmit Human Malaria
- PMID: 27490374
- PMCID: PMC4973987
- DOI: 10.1371/journal.ppat.1005773
Plant-Mediated Effects on Mosquito Capacity to Transmit Human Malaria
Abstract
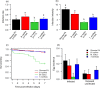
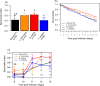
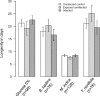
The ecological context in which mosquitoes and malaria parasites interact has received little attention, compared to the genetic and molecular aspects of malaria transmission. Plant nectar and fruits are important for the nutritional ecology of malaria vectors, but how the natural diversity of plant-derived sugar sources affects mosquito competence for malaria parasites is unclear. To test this, we infected Anopheles coluzzi, an important African malaria vector, with sympatric field isolates of Plasmodium falciparum, using direct membrane feeding assays. Through a series of experiments, we then examined the effects of sugar meals from Thevetia neriifolia and Barleria lupilina cuttings that included flowers, and fruit from Lannea microcarpa and Mangifera indica on parasite and mosquito traits that are key for determining the intensity of malaria transmission. We found that the source of plant sugar meal differentially affected infection prevalence and intensity, the development duration of the parasites, as well as the survival and fecundity of the vector. These effects are likely the result of complex interactions between toxic secondary metabolites and the nutritional quality of the plant sugar source, as well as of host resource availability and parasite growth. Using an epidemiological model, we show that plant sugar source can be a significant driver of malaria transmission dynamics, with some plant species exhibiting either transmission-reducing or -enhancing activities.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Contrasting effects of the alkaloid ricinine on the capacity of Anopheles gambiae and Anopheles coluzzii to transmit Plasmodium falciparum.Parasit Vectors. 2021 Sep 15;14(1):479. doi: 10.1186/s13071-021-04992-z. Parasit Vectors. 2021. PMID: 34526119 Free PMC article.
-
Additional Feeding Reveals Differences in Immune Recognition and Growth of Plasmodium Parasites in the Mosquito Host.mSphere. 2021 Mar 31;6(2):e00136-21. doi: 10.1128/mSphere.00136-21. mSphere. 2021. PMID: 33789941 Free PMC article.
-
Blood meal profile and positivity rate with malaria parasites among different malaria vectors in Sudan.Malar J. 2022 Apr 15;21(1):124. doi: 10.1186/s12936-022-04157-y. Malar J. 2022. PMID: 35428264 Free PMC article.
-
[Biology of man-mosquito Plasmodium transmission].Bull Soc Pathol Exot. 2003 Mar;96(1):6-20. Bull Soc Pathol Exot. 2003. PMID: 12784587 Review. French.
-
[Plasmodium falciparum: epidemiology and man-mosquito transmission and infection in the vector].Bull Soc Pathol Exot. 2003 Nov;96(4):335-40. Bull Soc Pathol Exot. 2003. PMID: 14717055 Review. French.
Cited by
-
Ecology of reproduction of Anopheles arabiensis in an urban area of Bobo-Dioulasso, Burkina Faso (West Africa): Monthly swarming and mating frequency and their relation to environmental factors.PLoS One. 2018 Nov 7;13(11):e0205966. doi: 10.1371/journal.pone.0205966. eCollection 2018. PLoS One. 2018. PMID: 30403762 Free PMC article.
-
Contemporary exploitation of natural products for arthropod-borne pathogen transmission-blocking interventions.Parasit Vectors. 2022 Aug 24;15(1):298. doi: 10.1186/s13071-022-05367-8. Parasit Vectors. 2022. PMID: 36002857 Free PMC article. Review.
-
Putting evolution in elimination: Winning our ongoing battle with evolving malaria mosquitoes and parasites.Evol Appl. 2017 Nov 6;11(4):415-430. doi: 10.1111/eva.12530. eCollection 2018 Apr. Evol Appl. 2017. PMID: 29636796 Free PMC article. Review.
-
Behavioural and Electrophysiological Responses of Female Anopheles gambiae Mosquitoes to Volatiles from a Mango Bait.J Chem Ecol. 2020 Apr;46(4):387-396. doi: 10.1007/s10886-020-01172-8. Epub 2020 Apr 9. J Chem Ecol. 2020. PMID: 32274623 Free PMC article.
-
Glucose transporter GLUT1 influences Plasmodium berghei infection in Anopheles stephensi.Parasit Vectors. 2020 Jun 5;13(1):285. doi: 10.1186/s13071-020-04155-6. Parasit Vectors. 2020. PMID: 32503601 Free PMC article.
References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

