O-linked GlcNAcylation elevated by HPV E6 mediates viral oncogenesis
- PMID: 27482104
- PMCID: PMC4995993
- DOI: 10.1073/pnas.1606801113
O-linked GlcNAcylation elevated by HPV E6 mediates viral oncogenesis
Abstract
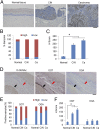
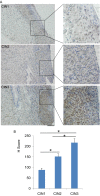
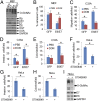
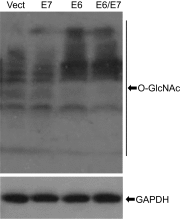
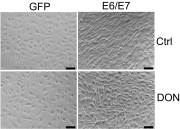
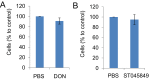
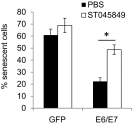
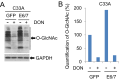
High-risk human papillomaviruses (HPVs) are causative agents of anogenital cancers and a fraction of head and neck cancers. The mechanisms involved in the progression of HPV neoplasias to cancers remain largely unknown. Here, we report that O-linked GlcNAcylation (O-GlcNAc) and O-GlcNAc transferase (OGT) were markedly increased in HPV-caused cervical neoplasms relative to normal cervix, whereas O-GlcNAcase (OGA) levels were not altered. Transduction of HPV16 oncogene E6 or E6/E7 into mouse embryonic fibroblasts (MEFs) up-regulated OGT mRNA and protein, elevated the level of O-GlcNAc, and promoted cell proliferation while reducing cellular senescence. Conversely, in HPV-18-transformed HeLa cervical carcinoma cells, inhibition of O-GlcNAc with a low concentration of a chemical inhibitor impaired the transformed phenotypes in vitro. We showed that E6 elevated c-MYC via increased protein stability attributable to O-GlcNAcylation on Thr58. Reduction of HPV-mediated cell viability by a high concentration of O-GlcNAc inhibitor was partially rescued by elevated c-MYC. Finally, knockdown of OGT or O-GlcNAc inhibition in HeLa cells or in TC-1 cells, a mouse cell line transformed by HPV16 E6/E7 and activated K-RAS, reduced c-MYC and suppressed tumorigenesis and metastasis. Thus, we have uncovered a mechanism for HPV oncoprotein-mediated transformation. These findings may eventually aid in the development of effective therapeutics for HPV-associated malignancies by targeting aberrant O-GlcNAc.
Keywords: HPV E6; HPV oncogenicity; O-linked GlcNAcylation; c-MYC; cervical cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
O-linked N-acetylglucosamine transferase promotes cervical cancer tumorigenesis through human papillomaviruses E6 and E7 oncogenes.Oncotarget. 2016 Jul 12;7(28):44596-44607. doi: 10.18632/oncotarget.10112. Oncotarget. 2016. PMID: 27331873 Free PMC article.
-
O-linked-N-acetylglucosamine transferase is associated with metastatic spread of human papillomavirus E6 and E7 oncoproteins to the lungs of mice.Biochem Biophys Res Commun. 2017 Feb 5;483(2):793-802. doi: 10.1016/j.bbrc.2016.10.156. Epub 2016 Nov 12. Biochem Biophys Res Commun. 2017. PMID: 27845045
-
Honeybee venom possesses anticancer and antiviral effects by differential inhibition of HPV E6 and E7 expression on cervical cancer cell line.Oncol Rep. 2015 Apr;33(4):1675-82. doi: 10.3892/or.2015.3760. Epub 2015 Jan 28. Oncol Rep. 2015. PMID: 25633640
-
[Molecular basis of cervical carcinogenesis by high-risk human papillomaviruses].Uirusu. 2008 Dec;58(2):141-54. doi: 10.2222/jsv.58.141. Uirusu. 2008. PMID: 19374192 Review. Japanese.
-
Basic mechanisms of high-risk human papillomavirus-induced carcinogenesis: roles of E6 and E7 proteins.Cancer Sci. 2007 Oct;98(10):1505-11. doi: 10.1111/j.1349-7006.2007.00546.x. Epub 2007 Jul 23. Cancer Sci. 2007. PMID: 17645777 Free PMC article. Review.
Cited by
-
Recent findings in the regulation of G6PD and its role in diseases.Front Pharmacol. 2022 Aug 24;13:932154. doi: 10.3389/fphar.2022.932154. eCollection 2022. Front Pharmacol. 2022. PMID: 36091812 Free PMC article. Review.
-
Metabolic reprogramming in cervical cancer and metabolomics perspectives.Nutr Metab (Lond). 2021 Oct 19;18(1):93. doi: 10.1186/s12986-021-00615-7. Nutr Metab (Lond). 2021. PMID: 34666780 Free PMC article. Review.
-
O-GlcNAcylation links oncogenic signals and cancer epigenetics.Discov Oncol. 2021 Nov 24;12(1):54. doi: 10.1007/s12672-021-00450-5. Discov Oncol. 2021. PMID: 35201498 Free PMC article. Review.
-
O-GlcNAcylation and Its Role in Cancer-Associated Inflammation.Front Immunol. 2022 Apr 1;13:861559. doi: 10.3389/fimmu.2022.861559. eCollection 2022. Front Immunol. 2022. PMID: 35432358 Free PMC article. Review.
-
Differential expression of mRNA 3'-end isoforms in cervical and ovarian cancers.Heliyon. 2023 Sep 9;9(9):e20035. doi: 10.1016/j.heliyon.2023.e20035. eCollection 2023 Sep. Heliyon. 2023. PMID: 37810050 Free PMC article.
References
-
- Walboomers JM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–19. - PubMed
-
- zur Hausen H. Papillomaviruses in the causation of human cancers: A brief historical account. Virology. 2009;384(2):260–265. - PubMed
-
- Lajer CB, von Buchwald C. The role of human papillomavirus in head and neck cancer. APMIS. 2010;118(6-7):510–519. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

