Non-canonical Interactions between Heat Shock Cognate Protein 70 (Hsc70) and Bcl2-associated Anthanogene (BAG) Co-Chaperones Are Important for Client Release
- PMID: 27474739
- PMCID: PMC5025674
- DOI: 10.1074/jbc.M116.742502
Non-canonical Interactions between Heat Shock Cognate Protein 70 (Hsc70) and Bcl2-associated Anthanogene (BAG) Co-Chaperones Are Important for Client Release
Abstract
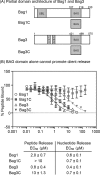
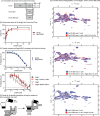
Heat shock cognate protein 70 (Hsc70) regulates protein homeostasis through its reversible interactions with client proteins. Hsc70 has two major domains: a nucleotide-binding domain (NBD), that hydrolyzes ATP, and a substrate-binding domain (SBD), where clients are bound. Members of the BAG family of co-chaperones, including Bag1 and Bag3, are known to accelerate release of both ADP and client from Hsc70. The release of nucleotide is known to be mediated by interactions between the conserved BAG domain and the Hsc70 NBD. However, less is known about the regions required for client release, and it is often assumed that this activity also requires the BAG domain. It is important to better understand this step because it determines how long clients remain in the inactive, bound state. Here, we report the surprising observation that truncated versions of either human Bag1 or Bag3, comprised only the BAG domain, promoted rapid release of nucleotide, but not client, in vitro Rather, we found that a non-canonical interaction between Bag1/3 and the Hsc70 SBD is sufficient for accelerating this step. Moreover, client release did not seem to require the BAG domain or Hsc70 NBD. These results suggest that Bag1 and Bag3 control the stability of the Hsc70-client complex using at least two distinct protein-protein contacts, providing a previously under-appreciated layer of molecular regulation in the human Hsc70 system.
Keywords: 70 kilodalton heat shock protein (Hsp70); molecular chaperone; nuclear magnetic resonance (NMR); protein complex; protein-protein interaction.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


Similar articles
-
Binding of human nucleotide exchange factors to heat shock protein 70 (Hsp70) generates functionally distinct complexes in vitro.J Biol Chem. 2014 Jan 17;289(3):1402-14. doi: 10.1074/jbc.M113.521997. Epub 2013 Dec 5. J Biol Chem. 2014. PMID: 24318877 Free PMC article.
-
Structural analysis of BAG1 cochaperone and its interactions with Hsc70 heat shock protein.Nat Struct Biol. 2001 Apr;8(4):349-52. doi: 10.1038/86236. Nat Struct Biol. 2001. PMID: 11276257
-
HSC70-JNK-BAG3 complex is critical for cardiomyocyte protection of BAG3 through its PXXP and BAG structural domains.Front Biosci (Landmark Ed). 2021 May 30;26(6):102-113. doi: 10.52586/4927. Front Biosci (Landmark Ed). 2021. PMID: 34162039
-
Breaking BAG: The Co-Chaperone BAG3 in Health and Disease.Trends Pharmacol Sci. 2016 Aug;37(8):672-688. doi: 10.1016/j.tips.2016.04.007. Epub 2016 May 6. Trends Pharmacol Sci. 2016. PMID: 27162137 Review.
-
Regulation of the heat shock conjugate Hsc70 in the mammalian cell: the characterization of the anti-apoptotic protein BAG-1 provides novel insights.Biol Chem. 1998 Mar;379(3):269-74. Biol Chem. 1998. PMID: 9563821 Review.
Cited by
-
Long noncoding RNA MAGI2-AS3 regulates the H2O2 level and cell senescence via HSPA8.Redox Biol. 2022 Aug;54:102383. doi: 10.1016/j.redox.2022.102383. Epub 2022 Jun 30. Redox Biol. 2022. PMID: 35797800 Free PMC article.
-
X-linked inhibitor of apoptosis protein (XIAP) is a client of heat shock protein 70 (Hsp70) and a biomarker of its inhibition.J Biol Chem. 2018 Feb 16;293(7):2370-2380. doi: 10.1074/jbc.RA117.000634. Epub 2017 Dec 18. J Biol Chem. 2018. PMID: 29255093 Free PMC article.
-
Liver Transcriptome Response to Heat Stress in Beijing You Chickens and Guang Ming Broilers.Genes (Basel). 2022 Feb 25;13(3):416. doi: 10.3390/genes13030416. Genes (Basel). 2022. PMID: 35327970 Free PMC article.
-
Multivalent protein-protein interactions are pivotal regulators of eukaryotic Hsp70 complexes.Cell Stress Chaperones. 2022 Jul;27(4):397-415. doi: 10.1007/s12192-022-01281-1. Epub 2022 Jun 7. Cell Stress Chaperones. 2022. PMID: 35670950 Free PMC article. Review.
-
Potentiating Therapeutic Effects of Epidermal Growth Factor Receptor Inhibition in Triple-Negative Breast Cancer.Pharmaceuticals (Basel). 2021 Jun 18;14(6):589. doi: 10.3390/ph14060589. Pharmaceuticals (Basel). 2021. PMID: 34207383 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

