Polyfunctional Melan-A-specific tumor-reactive CD8(+) T cells elicited by dacarbazine treatment before peptide-vaccination depends on AKT activation sustained by ICOS
- PMID: 27467927
- PMCID: PMC4910730
- DOI: 10.1080/2162402X.2015.1114203
Polyfunctional Melan-A-specific tumor-reactive CD8(+) T cells elicited by dacarbazine treatment before peptide-vaccination depends on AKT activation sustained by ICOS
Abstract
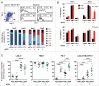
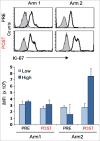
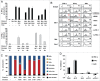
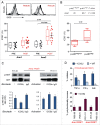
The identification of activation pathways linked to antitumor T-cell polyfunctionality in long surviving patients is of great relevance in the new era of immunotherapy. We have recently reported that dacarbazine (DTIC) injected one day before peptide-vaccination plus IFN-α improves the antitumor lytic activity and enlarges the repertoire of Melan-A-specific T-cell clones, as compared with vaccination alone, impacting the overall survival of melanoma patients. To identify the mechanisms responsible for this improvement of the immune response, we have analyzed the endogenous and treatment-induced antigen (Ag)-specific response in a panel of Melan-A-specific CD8(+) T-cell clones in terms of differentiation phenotype, inhibitory receptor profile, polyfunctionality and AKT activation. Here, we show that Melan-A-specific CD8(+) T cells isolated from patients treated with chemoimmunotherapy possess a late differentiated phenotype as defined by the absence of CD28 and CD27 co-stimulatory molecules and high levels of LAG-3, TIM-3 and PD-1 inhibitory receptors. Nevertheless, they show higher proliferative potential and an improved antitumor polyfunctional effector profile in terms of co-production of TNF-α, IFNγ and Granzyme-B (GrB) compared with cells derived from patients treated with vaccination alone. Polyfunctionality is dependent on an active AKT signaling related to the engagement of the co-stimulatory molecule ICOS. We suggest that this phenotypic and functional signature is dictated by a fine-tuned balance between TCR triggering, AKT activation, co-stimulatory and inhibitory signals induced by chemoimmunotherapy and may be associated with antitumor T cells able to protect patients from tumor recurrence.
Keywords: AKT; CD8+ T cells; ICOS; Melan-A; chemoimmunotherapy; dacarbazine; human melanoma; polyfunctional.
Figures


Similar articles
-
Antigen-specificity and DTIC before peptide-vaccination differently shape immune-checkpoint expression pattern, anti-tumor functionality and TCR repertoire in melanoma patients.Oncoimmunology. 2018 Sep 11;7(12):e1465163. doi: 10.1080/2162402X.2018.1465163. eCollection 2018. Oncoimmunology. 2018. PMID: 30524882 Free PMC article.
-
Dacarbazine treatment before peptide vaccination enlarges T-cell repertoire diversity of melan-a-specific, tumor-reactive CTL in melanoma patients.Cancer Res. 2010 Sep 15;70(18):7084-92. doi: 10.1158/0008-5472.CAN-10-1326. Epub 2010 Sep 7. Cancer Res. 2010. PMID: 20823160
-
Selective responsiveness to common gamma chain cytokines in peripheral blood-derived cytotoxic T lymphocytes induced by Melan-A/MART-1(27-35)targeted active specific immunotherapy.Int J Cancer. 2005 Jun 10;115(2):248-55. doi: 10.1002/ijc.20858. Int J Cancer. 2005. PMID: 15688403
-
Peptide-specific CD8+ T-cell evolution in vivo: response to peptide vaccination with Melan-A/MART-1.Int J Cancer. 2002 Mar 20;98(3):376-88. doi: 10.1002/ijc.10165. Int J Cancer. 2002. PMID: 11920589
-
Immune modulations during chemoimmunotherapy & novel vaccine strategies--in metastatic melanoma and non small-cell lung cancer.Dan Med J. 2013 Dec;60(12):B4774. Dan Med J. 2013. PMID: 24355457 Review.
Cited by
-
Polymorphic Region-Specific Antibody for Evaluation of Affinity-Associated Profile of Chimeric Antigen Receptor.Mol Ther Oncolytics. 2020 Apr 14;17:293-305. doi: 10.1016/j.omto.2020.04.004. eCollection 2020 Jun 26. Mol Ther Oncolytics. 2020. PMID: 32368617 Free PMC article.
-
Blood immune cells as potential biomarkers predicting relapse-free survival of stage III/IV resected melanoma patients treated with peptide-based vaccination and interferon-alpha.Front Oncol. 2023 May 18;13:1145667. doi: 10.3389/fonc.2023.1145667. eCollection 2023. Front Oncol. 2023. PMID: 37274275 Free PMC article.
-
Altered Peptide Ligands Impact the Diversity of Polyfunctional Phenotypes in T Cell Receptor Gene-Modified T Cells.Mol Ther. 2018 Apr 4;26(4):996-1007. doi: 10.1016/j.ymthe.2018.01.015. Epub 2018 Feb 2. Mol Ther. 2018. PMID: 29503203 Free PMC article.
-
Single-cell multiplexed cytokine profiling of CD19 CAR-T cells reveals a diverse landscape of polyfunctional antigen-specific response.J Immunother Cancer. 2017 Nov 21;5(1):85. doi: 10.1186/s40425-017-0293-7. J Immunother Cancer. 2017. PMID: 29157295 Free PMC article.
-
Advances in Immunotherapy for Melanoma: A Comprehensive Review.Mediators Inflamm. 2017;2017:3264217. doi: 10.1155/2017/3264217. Epub 2017 Aug 1. Mediators Inflamm. 2017. PMID: 28848246 Free PMC article. Review.
References
-
- Nisticò P, Capone I, Palermo B, Del Bello D, Ferraresi V, Moschella F, Aricò E, Valentini M, Bracci L, Cognetti F et al.. Chemotherapy enhances vaccine-induced antitumor immunity in melanoma patients. Int J Cancer 2009; 124:130-9; PMID:18839429; http://dx.doi.org/2082316010.1002/ijc.23886 - DOI - PubMed
-
- Palermo B, Del Bello D, Sottini A, Serana F, Ghidini C, Gualtieri N, Ferraresi V, Catricalà C, Belardelli F, Proietti E et al.. Dacarbazine treatment before peptide vaccination enlarges T-cell repertoire diversity of melan-a-specific, tumor-reactive CTL in melanoma patients. Cancer Res 2010; 70:7084-92; PMID:20823160; http://dx.doi.org/10.1158/0008-5472.CAN-10-1326 - DOI - PubMed
-
- Frauwirth KA, Thompson CB. Activation and inhibition of lymphocytes by costimulation. J Clin Invest 2002; 109:295-299; PMID:11827987; http://dx.doi.org/10.1172/JCI0214941 - DOI - PMC - PubMed
-
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12:252-64; PMID:22437870; http://dx.doi.org/10.1038/nrc3239 - DOI - PMC - PubMed
-
- Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol 1996; 14:233-58; PMID:8717514; http://dx.doi.org/10.1146/annurev.immunol.14.1.233 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
