Engineered Mesenchymal Stem Cells as an Anti-Cancer Trojan Horse
- PMID: 27460260
- PMCID: PMC5035934
- DOI: 10.1089/scd.2016.0120
Engineered Mesenchymal Stem Cells as an Anti-Cancer Trojan Horse
Abstract
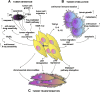
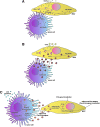
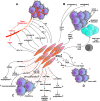
Cell-based gene therapy holds a great promise for the treatment of human malignancy. Among different cells, mesenchymal stem cells (MSCs) are emerging as valuable anti-cancer agents that have the potential to be used to treat a number of different cancer types. They have inherent migratory properties, which allow them to serve as vehicles for delivering effective therapy to isolated tumors and metastases. MSCs have been engineered to express anti-proliferative, pro-apoptotic, and anti-angiogenic agents that specifically target different cancers. Another field of interest is to modify MSCs with the cytokines that activate pro-tumorigenic immunity or to use them as carriers for the traditional chemical compounds that possess the properties of anti-cancer drugs. Although there is still controversy about the exact function of MSCs in the tumor settings, the encouraging results from the preclinical studies of MSC-based gene therapy for a large number of tumors support the initiation of clinical trials.
Conflict of interest statement
Author Disclosure Statement All authors declare that no competing financial interests exist.
Figures
Similar articles
-
Revolutionizing Cancer Treatment: Harnessing the Power of Mesenchymal Stem Cells for Precise Targeted Therapy in the Tumor Microenvironment.Curr Top Med Chem. 2024 May 23. doi: 10.2174/0115680266299112240514103048. Online ahead of print. Curr Top Med Chem. 2024. PMID: 38797895
-
Mesenchymal stem cells engineered for cancer therapy.Adv Drug Deliv Rev. 2012 Jun 1;64(8):739-48. doi: 10.1016/j.addr.2011.06.010. Epub 2011 Jun 29. Adv Drug Deliv Rev. 2012. PMID: 21740940 Free PMC article. Review.
-
Current progress in engineered and nano-engineered mesenchymal stem cells for cancer: From mechanisms to therapy.Biomed Pharmacother. 2023 Nov;167:115505. doi: 10.1016/j.biopha.2023.115505. Epub 2023 Sep 15. Biomed Pharmacother. 2023. PMID: 37716113 Review.
-
How desirable and undesirable features of naïve or genetically reengineered mesenchymal stem cells are being considered in preclinical or clinical assays.J BUON. 2017 Jul-Aug;22(4):812-830. J BUON. 2017. PMID: 29155506 Review.
-
Selective targeting of genetically engineered mesenchymal stem cells to tumor stroma microenvironments using tissue-specific suicide gene expression suppresses growth of hepatocellular carcinoma.Ann Surg. 2011 Nov;254(5):767-74; discussion 774-5. doi: 10.1097/SLA.0b013e3182368c4f. Ann Surg. 2011. PMID: 22042469
Cited by
-
Mesenchymal Stem Cell Expressing TRAIL as Targeted Therapy against Sensitised Tumour.Int J Mol Sci. 2018 Jul 27;19(8):2188. doi: 10.3390/ijms19082188. Int J Mol Sci. 2018. PMID: 30060445 Free PMC article. Review.
-
Melatonin and Mesenchymal Stem Cells as a Key for Functional Integrity for Liver Cancer Treatment.Int J Mol Sci. 2020 Jun 25;21(12):4521. doi: 10.3390/ijms21124521. Int J Mol Sci. 2020. PMID: 32630505 Free PMC article. Review.
-
Mesenchymal stromal cells as carriers of IL-12 reduce primary and metastatic tumors of murine melanoma.Sci Rep. 2021 Sep 15;11(1):18335. doi: 10.1038/s41598-021-97435-9. Sci Rep. 2021. PMID: 34526531 Free PMC article.
-
Bioengineered Mesenchymal Stem/Stromal Cells in Anti-Cancer Therapy: Current Trends and Future Prospects.Biomolecules. 2024 Jun 21;14(7):734. doi: 10.3390/biom14070734. Biomolecules. 2024. PMID: 39062449 Free PMC article. Review.
-
Concise Review: Mesenchymal Stem Cells: From Roots to Boost.Stem Cells. 2019 Jul;37(7):855-864. doi: 10.1002/stem.3016. Epub 2019 Apr 30. Stem Cells. 2019. PMID: 30977255 Free PMC article. Review.
References
-
- Nowakowski A, Walczak P, Janowski M. and Lukomska B. (2015). Genetic engineering of mesenchymal stem cells for regenerative medicine. Stem Cells Dev 24:2219–2242 - PubMed
-
- Nowakowski A, Andrzejewska A, Janowski M, Walczak P. and Lukomska B. (2013). Genetic engineering of stem cells for enhanced therapy. Acta Neurobiol Exp (Wars) 73:1–18 - PubMed
-
- Barcellos-de-Souza P, Gori V, Bambi F. and Chiarugi P. (2013). Tumor microenvironment: bone marrow-mesenchymal stem cells as key players. Biochim Biophys Acta 1836:321–335 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

