High Morphologic Plasticity of Microglia/Macrophages Following Experimental Intracerebral Hemorrhage in Rats
- PMID: 27455236
- PMCID: PMC4964551
- DOI: 10.3390/ijms17071181
High Morphologic Plasticity of Microglia/Macrophages Following Experimental Intracerebral Hemorrhage in Rats
Abstract
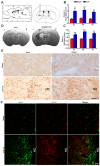
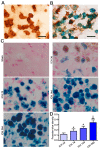
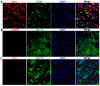
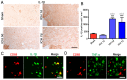
As current efforts have limited effects on the clinical outcome of intracerebral hemorrhage (ICH), the mechanisms including microglia/macrophages that involved inflammation need further investigation. Here, 0.4 units of collagenase VII were injected into the left caudate putamen (CPu) to duplicate ICH rat models. In the brains of ICH rats, microglia/macrophages, the nearest cells to the hemorrhagic center, were observed as ameboid and Prussian-blue positive. Furthermore, the ameboid microglia/macrophages were differentiation (CD) 68 and interleukin-1β (IL-1β) positive, and neither CD206 nor chitinase3-like 3 (Ym1) positive, suggesting their strong abilities of phagocytosis and secretion of IL-1β. According to the distance to the hemorrhagic center, we selected four areas-I, II, III, and IV-to analyze the morphology of microglia/macrophages. The processes decreased successively from region I to region IV. Microglia/macrophages in region IV had no processes. The processes in region I were radially distributed, however, they showed obvious directivity towards the hemorrhagic center in regions II and III. Region III had the largest density of compactly arrayed microglia/macrophages. All these in vivo results present the high morphologic plasticity of microglia/macrophages and their functions in the pathogenesis of ICHs.
Keywords: interleukin-10; interleukin-1β; intracerebral hemorrhage; microglia.
Figures
Similar articles
-
Functions of lactate in the brain of rat with intracerebral hemorrhage evaluated with MRI/MRS and in vitro approaches.CNS Neurosci Ther. 2020 Oct;26(10):1031-1044. doi: 10.1111/cns.13399. Epub 2020 Jun 2. CNS Neurosci Ther. 2020. PMID: 32488963 Free PMC article.
-
Rapamycin protects against neuronal death and improves neurological function with modulation of microglia after experimental intracerebral hemorrhage in rats.Cell Mol Biol (Noisy-le-grand). 2016 Sep 30;62(11):67-75. Cell Mol Biol (Noisy-le-grand). 2016. PMID: 27755955
-
Edaravone Administration Confers Neuroprotection after Experimental Intracerebral Hemorrhage in Rats via NLRP3 Suppression.J Stroke Cerebrovasc Dis. 2020 Jan;29(1):104468. doi: 10.1016/j.jstrokecerebrovasdis.2019.104468. Epub 2019 Nov 4. J Stroke Cerebrovasc Dis. 2020. PMID: 31694784
-
Increased expression of T cell immunoglobulin and mucin domain 3 aggravates brain inflammation via regulation of the function of microglia/macrophages after intracerebral hemorrhage in mice.J Neuroinflammation. 2013 Dec 1;10:141. doi: 10.1186/1742-2094-10-141. J Neuroinflammation. 2013. PMID: 24289479 Free PMC article.
-
Alternative activation-skewed microglia/macrophages promote hematoma resolution in experimental intracerebral hemorrhage.Neurobiol Dis. 2017 Jul;103:54-69. doi: 10.1016/j.nbd.2017.03.016. Epub 2017 Mar 30. Neurobiol Dis. 2017. PMID: 28365213 Free PMC article.
Cited by
-
Updates of the role of B-cells in ischemic stroke.Front Cell Neurosci. 2024 Mar 14;18:1340756. doi: 10.3389/fncel.2024.1340756. eCollection 2024. Front Cell Neurosci. 2024. PMID: 38550918 Free PMC article. Review.
-
A modified impactor for establishing a graded contusion spinal cord injury model in rats.Ann Transl Med. 2022 Apr;10(8):436. doi: 10.21037/atm-21-5851. Ann Transl Med. 2022. PMID: 35571430 Free PMC article.
-
Emodin Prevented Depression in Chronic Unpredicted Mild Stress-Exposed Rats by Targeting miR-139-5p/5-Lipoxygenase.Front Cell Dev Biol. 2021 Jul 26;9:696619. doi: 10.3389/fcell.2021.696619. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34381778 Free PMC article.
-
Synthesis and Development of a Novel First-in-Class Cofilin Inhibitor for Neuroinflammation in Hemorrhagic Brain Injury.ACS Chem Neurosci. 2022 Apr 6;13(7):1014-1029. doi: 10.1021/acschemneuro.2c00010. Epub 2022 Mar 18. ACS Chem Neurosci. 2022. PMID: 35302736 Free PMC article.
-
Unraveling the complex pathophysiology of white matter hemorrhage in intracerebral stroke: A single-cell RNA sequencing approach.CNS Neurosci Ther. 2024 Mar;30(3):e14652. doi: 10.1111/cns.14652. CNS Neurosci Ther. 2024. PMID: 38433011 Free PMC article.
References
-
- Krishnamurthi R.V., Moran A.E., Forouzanfar M.H., Bennett D.A., Mensah G.A., Lawes C.M., Barker-Collo S., Connor M., Roth G.A., Sacco R., et al. Global Burden of Diseases, Injuries, and Risk Factors 2010 Study Stroke Expert Group, The global burden of hemorrhagic stroke: A summary of findings from the GBD 2010 study. Glob. Heart. 2014;9:101–106. doi: 10.1016/j.gheart.2014.01.003. - DOI - PubMed
-
- O’Donnell M.J., Xavier D., Liu L., Zhang H., Chin S.L., Rao-Melacini P., Rangarajan S., Islam S., Pais P., McQueen M.J., et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the Interstroke study): A case-control study. Lancet. 2010;376:112–123. doi: 10.1016/S0140-6736(10)60834-3. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

