In Brief: Mitophagy: mechanisms and role in human disease
- PMID: 27453450
- PMCID: PMC5071152
- DOI: 10.1002/path.4774
In Brief: Mitophagy: mechanisms and role in human disease
Abstract
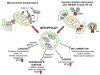
Mitophagy is a selective form of macro-autophagy in which mitochondria are specifically targeted for autophagic degradation. Mitophagy plays an important role in cellular homeostasis by eliminating dysfunctional mitochondria and reducing mitochondrial mass as an adaptive response to stress. Cells execute mitophagy through several non-redundant mechanisms, including the PINK1/Parkin partnership, which modulates turnover of depolarized mitochondria, and stress-induced BNIP3, NIX, and FUNDC1 molecular adaptors, which interact directly with LC3 to promote mitophagy. These pathways are deregulated in human diseases, including cancer, neurodegeneration, metabolic disorders, muscle atrophy, ageing, and inflammation, reflecting the importance of mitophagy as a cellular housekeeping function. Copyright © 2016 Pathological Society of Great Britain and Ireland. Published by John Wiley & Sons, Ltd.
Keywords: BNIP3; FUNDC1; NIX; PINK1; Parkin; ageing; autophagy; disease; mitochondria.
Copyright © 2016 Pathological Society of Great Britain and Ireland. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
Similar articles
-
Nix restores mitophagy and mitochondrial function to protect against PINK1/Parkin-related Parkinson's disease.Sci Rep. 2017 Mar 10;7:44373. doi: 10.1038/srep44373. Sci Rep. 2017. PMID: 28281653 Free PMC article.
-
Mitophagy programs: mechanisms and physiological implications of mitochondrial targeting by autophagy.Cell Mol Life Sci. 2016 Feb;73(4):775-95. doi: 10.1007/s00018-015-2087-8. Epub 2015 Nov 26. Cell Mol Life Sci. 2016. PMID: 26611876 Free PMC article. Review.
-
FKBP8 recruits LC3A to mediate Parkin-independent mitophagy.EMBO Rep. 2017 Jun;18(6):947-961. doi: 10.15252/embr.201643147. Epub 2017 Apr 5. EMBO Rep. 2017. PMID: 28381481 Free PMC article.
-
Role of BNIP3 and NIX in cell death, autophagy, and mitophagy.Cell Death Differ. 2009 Jul;16(7):939-46. doi: 10.1038/cdd.2009.16. Epub 2009 Feb 20. Cell Death Differ. 2009. PMID: 19229244 Free PMC article. Review.
-
A brief overview of BNIP3L/NIX receptor-mediated mitophagy.FEBS Open Bio. 2021 Dec;11(12):3230-3236. doi: 10.1002/2211-5463.13307. Epub 2021 Oct 11. FEBS Open Bio. 2021. PMID: 34597467 Free PMC article. Review.
Cited by
-
Protective effect of resveratrol on rat cardiomyocyte H9C2 cells injured by hypoxia/reoxygenation by regulating mitochondrial autophagy PTEN-induced putative kinase protein 1/Parkinson disease protein 2 signaling pathway.J Tradit Chin Med. 2022 Apr;42(2):176-186. doi: 10.19852/j.cnki.jtcm.20220311.002. J Tradit Chin Med. 2022. PMID: 35473337 Free PMC article.
-
Acetoacetate protects macrophages from lactic acidosis-induced mitochondrial dysfunction by metabolic reprograming.Nat Commun. 2021 Dec 8;12(1):7115. doi: 10.1038/s41467-021-27426-x. Nat Commun. 2021. PMID: 34880237 Free PMC article.
-
Mechanism of Mitophagy and Its Role in Sepsis Induced Organ Dysfunction: A Review.Front Cell Dev Biol. 2021 Jun 7;9:664896. doi: 10.3389/fcell.2021.664896. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34164394 Free PMC article. Review.
-
The effect of fasting or calorie restriction on mitophagy induction: a literature review.J Cachexia Sarcopenia Muscle. 2020 Dec;11(6):1447-1458. doi: 10.1002/jcsm.12611. Epub 2020 Aug 27. J Cachexia Sarcopenia Muscle. 2020. PMID: 32856431 Free PMC article. Review.
-
The Role of PTEN-L in Modulating PINK1-Parkin-Mediated Mitophagy.Neurotox Res. 2022 Aug;40(4):1103-1114. doi: 10.1007/s12640-022-00475-w. Epub 2022 Jun 14. Neurotox Res. 2022. PMID: 35699891 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

