In Vivo therapeutic potential of mesenchymal stem cell-derived extracellular vesicles with optical imaging reporter in tumor mice model
- PMID: 27452924
- PMCID: PMC4958922
- DOI: 10.1038/srep30418
In Vivo therapeutic potential of mesenchymal stem cell-derived extracellular vesicles with optical imaging reporter in tumor mice model
Abstract
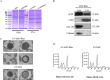
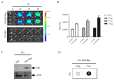
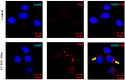
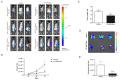
Mesenchymal stem cells (MSCs) can be used as a therapeutic armor for cancer. Extracellular vesicles (EVs) from MSCs have been evaluated for anticancer effects. In vivo targeting of EVs to the tumor is an essential requirement for successful therapy. Therefore, non-invasive methods of monitoring EVs in animal models are crucial for developing EV-based cancer therapies. The present study to develop bioluminescent EVs using Renilla luciferase (Rluc)-expressing MSCs. The EVs from MSC/Rluc cells (EV-MSC/Rluc) were visualized in a murine lung cancer model. The anticancer effects of EVs on Lewis lung carcinoma (LLC) and other cancer cells were assessed. EV-MSC/Rluc were visualized in vivo in the LLC-efffuc tumor model using optical imaging. The induction of apoptosis was confirmed with Annexin-V and propidium iodide staining. EV-MSC/Rluc and EV-MSCs showed a significant cytotoxic effect against LLC-effluc cells and 4T1; however, no significant effect on CT26, B16F10, TC1 cells. Moreover, EV-MSC/Rluc inhibited LLC tumor growth in vivo. EV-MSC/Rluc-mediated LLC tumor inhibitory mechanism revealed the decreased pERK and increased cleaved caspase 3 and cleaved PARP. We successfully developed luminescent EV-MSC/Rluc that have a therapeutic effect on LLC cells in both in vitro and in vivo. This bioluminescent EV system can be used to optimize EV-based therapy.
Figures

Similar articles
-
Regulated Mesenchymal Stem Cells Mediated Colon Cancer Therapy Assessed by Reporter Gene Based Optical Imaging.Int J Mol Sci. 2018 Mar 27;19(4):1002. doi: 10.3390/ijms19041002. Int J Mol Sci. 2018. PMID: 29584688 Free PMC article.
-
Migration of mesenchymal stem cells to tumor xenograft models and in vitro drug delivery by doxorubicin.Int J Med Sci. 2018 Jun 22;15(10):1051-1061. doi: 10.7150/ijms.25760. eCollection 2018. Int J Med Sci. 2018. PMID: 30013447 Free PMC article.
-
A new bioluminescent reporter system to study the biodistribution of systematically injected tumor-derived bioluminescent extracellular vesicles in mice.Oncotarget. 2017 Nov 18;8(66):109894-109914. doi: 10.18632/oncotarget.22493. eCollection 2017 Dec 15. Oncotarget. 2017. PMID: 29299117 Free PMC article.
-
Therapeutic roles of mesenchymal stem cell-derived extracellular vesicles in cancer.J Hematol Oncol. 2021 Sep 3;14(1):136. doi: 10.1186/s13045-021-01141-y. J Hematol Oncol. 2021. PMID: 34479611 Free PMC article. Review.
-
Mesenchymal Stem Cell Derived Extracellular Vesicles for Tissue Engineering and Regenerative Medicine Applications.Cells. 2020 Apr 16;9(4):991. doi: 10.3390/cells9040991. Cells. 2020. PMID: 32316248 Free PMC article. Review.
Cited by
-
Comparative Analysis of Natural and Cytochalasin B-Induced Membrane Vesicles from Tumor Cells and Mesenchymal Stem Cells.Curr Issues Mol Biol. 2022 Nov 1;44(11):5363-5378. doi: 10.3390/cimb44110363. Curr Issues Mol Biol. 2022. PMID: 36354675 Free PMC article.
-
Progress of Endogenous and Exogenous Nanoparticles for Cancer Therapy and Diagnostics.Genes (Basel). 2023 Jan 19;14(2):259. doi: 10.3390/genes14020259. Genes (Basel). 2023. PMID: 36833186 Free PMC article. Review.
-
Targeting and Therapy of Glioblastoma in a Mouse Model Using Exosomes Derived From Natural Killer Cells.Front Immunol. 2018 Apr 23;9:824. doi: 10.3389/fimmu.2018.00824. eCollection 2018. Front Immunol. 2018. Retraction in: Front Immunol. 2019 Jul 16;10:1770. doi: 10.3389/fimmu.2019.01770. PMID: 29740437 Free PMC article. Retracted.
-
Combined Role of Interleukin-15 Stimulated Natural Killer Cell-Derived Extracellular Vesicles and Carboplatin in Osimertinib-Resistant H1975 Lung Cancer Cells with EGFR Mutations.Pharmaceutics. 2024 Jan 8;16(1):83. doi: 10.3390/pharmaceutics16010083. Pharmaceutics. 2024. PMID: 38258094 Free PMC article.
-
On the Choice of the Extracellular Vesicles for Therapeutic Purposes.Int J Mol Sci. 2019 Jan 9;20(2):236. doi: 10.3390/ijms20020236. Int J Mol Sci. 2019. PMID: 30634425 Free PMC article. Review.
References
-
- Uccelli A., Moretta L. & Pistoia V. Mesenchymal stem cells in health and disease. Nat. Rev Immunol. 8, 726–736 (2008). - PubMed
-
- Bergfeld S. A. & DeClerck Y. A. Bone marrow-derived mesenchymal stem cells and the tumor microenvironment. Cancer. Metast Rev. 29, 249–261 (2010). - PubMed
-
- Ramasamy R. et al. Mesenchymal stem cells inhibit proliferation and apoptosis of tumor cells: impact on in vivo tumor growth. Leukemia. 21, 304–310 (2007). - PubMed
-
- Yu J. M., Jun E. S., Bae Y. C. & Jung J. S. Mesenchymal stem cells derived from human adipose tissues favor tumor cell growth in vivo. Stem Cells Dev. 17, 463–474 (2008). - PubMed
-
- Zhu W. et al. Mesenchymal stem cells derived from bone marrow favor tumor cell growth in vivo. Exp. Mol Pathol. 80, 267–274 (2006). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

