KSHV-Mediated Angiogenesis in Tumor Progression
- PMID: 27447661
- PMCID: PMC4974533
- DOI: 10.3390/v8070198
KSHV-Mediated Angiogenesis in Tumor Progression
Abstract
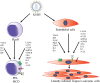
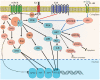
Human herpesvirus 8 (HHV-8), also known as Kaposi's sarcoma-associated herpesvirus (KSHV), is a malignant human oncovirus belonging to the gamma herpesvirus family. HHV-8 is closely linked to the pathogenesis of Kaposi's sarcoma (KS) and two other B-cell lymphoproliferative diseases: primary effusion lymphoma (PEL) and a plasmablastic variant of multicentric Castleman's disease (MCD). KS is an invasive tumor of endothelial cells most commonly found in untreated HIV-AIDS or immuno-compromised individuals. KS tumors are highly vascularized and have abnormal, excessive neo-angiogenesis, inflammation, and proliferation of infected endothelial cells. KSHV directly induces angiogenesis in an autocrine and paracrine fashion through a complex interplay of various viral and cellular pro-angiogenic and inflammatory factors. KS is believed to originate due to a combination of KSHV's efficient strategies for evading host immune systems and several pro-angiogenic and pro-inflammatory stimuli. In addition, KSHV infection of endothelial cells produces a wide array of viral oncoproteins with transforming capabilities that regulate multiple host-signaling pathways involved in the activation of angiogenesis. It is likely that the cellular-signaling pathways of angiogenesis and lymph-angiogenesis modulate the rate of tumorigenesis induction by KSHV. This review summarizes the current knowledge on regulating KSHV-mediated angiogenesis by integrating the findings reported thus far on the roles of host and viral genes in oncogenesis, recent developments in cell-culture/animal-model systems, and various anti-angiogenic therapies for treating KSHV-related lymphoproliferative disorders.
Keywords: KSHV; Kaposi’s sarcoma; Kaposi’s sarcoma-associated herpesvirus; angiogenesis; lymphangiogenesis; oncogenesis; oncoproteins.
Figures
Similar articles
-
Altering the Anti-inflammatory Lipoxin Microenvironment: a New Insight into Kaposi's Sarcoma-Associated Herpesvirus Pathogenesis.J Virol. 2016 Nov 28;90(24):11020-11031. doi: 10.1128/JVI.01491-16. Print 2016 Dec 15. J Virol. 2016. PMID: 27681120 Free PMC article.
-
Cyclooxygenase-2-prostaglandin E2-eicosanoid receptor inflammatory axis: a key player in Kaposi's sarcoma-associated herpes virus associated malignancies.Transl Res. 2013 Aug;162(2):77-92. doi: 10.1016/j.trsl.2013.03.004. Epub 2013 Apr 6. Transl Res. 2013. PMID: 23567332 Free PMC article. Review.
-
Kaposi's Sarcoma-Associated Herpesvirus Viral Interleukin-6 Signaling Upregulates Integrin β3 Levels and Is Dependent on STAT3.J Virol. 2020 Feb 14;94(5):e01384-19. doi: 10.1128/JVI.01384-19. Print 2020 Feb 14. J Virol. 2020. PMID: 31801855 Free PMC article.
-
KSHV induces aerobic glycolysis and angiogenesis through HIF-1-dependent upregulation of pyruvate kinase 2 in Kaposi's sarcoma.Angiogenesis. 2015 Oct;18(4):477-88. doi: 10.1007/s10456-015-9475-4. Epub 2015 Jun 20. Angiogenesis. 2015. PMID: 26092770 Free PMC article.
-
Kaposi's Sarcoma-Associated Herpesvirus: Epidemiology and Molecular Biology.Adv Exp Med Biol. 2017;1018:91-127. doi: 10.1007/978-981-10-5765-6_7. Adv Exp Med Biol. 2017. PMID: 29052134 Review.
Cited by
-
KSHV oral shedding and plasma viremia result in significant changes in the extracellular tumorigenic miRNA expression profile in individuals infected with the malaria parasite.PLoS One. 2018 Feb 9;13(2):e0192659. doi: 10.1371/journal.pone.0192659. eCollection 2018. PLoS One. 2018. PMID: 29425228 Free PMC article.
-
Human Gammaherpesvirus 8 Oncogenes Associated with Kaposi's Sarcoma.Int J Mol Sci. 2022 Jun 29;23(13):7203. doi: 10.3390/ijms23137203. Int J Mol Sci. 2022. PMID: 35806208 Free PMC article. Review.
-
DNA Tumor Virus Regulation of Host DNA Methylation and Its Implications for Immune Evasion and Oncogenesis.Viruses. 2018 Feb 13;10(2):82. doi: 10.3390/v10020082. Viruses. 2018. PMID: 29438328 Free PMC article. Review.
-
Overview of virus and cancer relationships. Position paper.Rev Esp Quimioter. 2021 Dec;34(6):525-555. doi: 10.37201/req/058.2021. Epub 2021 Aug 5. Rev Esp Quimioter. 2021. PMID: 34348449 Free PMC article.
-
Kaposi's sarcoma-associated herpesvirus infection promotes differentiation and polarization of monocytes into tumor-associated macrophages.Cell Cycle. 2017;16(17):1611-1621. doi: 10.1080/15384101.2017.1356509. Epub 2017 Jul 27. Cell Cycle. 2017. PMID: 28750175 Free PMC article.
References
-
- Sturzl M., Zietz C., Monini P., Ensoli B. Human herpesvirus-8 and kaposi’s sarcoma: Relationship with the multistep concept of tumorigenesis. Adv. Cancer Res. 2001;81:125–159. - PubMed
-
- Soulier J., Grollet L., Oksenhendler E., Cacoub P., Cazals-Hatem D., Babinet P., d’Agay M.F., Clauvel J.P., Raphael M., Degos L., et al. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric castleman’s disease. Blood. 1995;86:1276–1280. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

