Inducible Bronchus-Associated Lymphoid Tissue: Taming Inflammation in the Lung
- PMID: 27446088
- PMCID: PMC4928648
- DOI: 10.3389/fimmu.2016.00258
Inducible Bronchus-Associated Lymphoid Tissue: Taming Inflammation in the Lung
Abstract
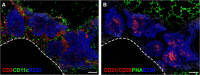
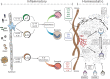
Following pulmonary inflammation, leukocytes that infiltrate the lung often assemble into structures known as inducible Bronchus-Associated Lymphoid Tissue (iBALT). Like conventional lymphoid organs, areas of iBALT have segregated B and T cell areas, specialized stromal cells, high endothelial venules, and lymphatic vessels. After inflammation is resolved, iBALT is maintained for months, independently of inflammation. Once iBALT is formed, it participates in immune responses to pulmonary antigens, including those that are unrelated to the iBALT-initiating antigen, and often alters the clinical course of disease. However, the mechanisms that govern immune responses in iBALT and determine how iBALT impacts local and systemic immunity are poorly understood. Here, we review our current understanding of iBALT formation and discuss how iBALT participates in pulmonary immunity.
Keywords: ectopic lymphoid organ; germinal center; inducible bronchus-associated lymphoid tissue; lymphoid neogenesis; tertiary lymphoid organ.
Figures
Similar articles
-
Role of iBALT in Respiratory Immunity.Curr Top Microbiol Immunol. 2020;426:21-43. doi: 10.1007/82_2019_191. Curr Top Microbiol Immunol. 2020. PMID: 31974759 Free PMC article. Review.
-
Inducible bronchus-associated lymphoid tissue (iBALT) in patients with pulmonary complications of rheumatoid arthritis.J Clin Invest. 2006 Dec;116(12):3183-94. doi: 10.1172/JCI28756. J Clin Invest. 2006. PMID: 17143328 Free PMC article.
-
Inducible Bronchus-Associated Lymphoid Tissue (iBALT) Attenuates Pulmonary Pathology in a Mouse Model of Allergic Airway Disease.Front Immunol. 2020 Sep 25;11:570661. doi: 10.3389/fimmu.2020.570661. eCollection 2020. Front Immunol. 2020. PMID: 33101290 Free PMC article.
-
Inducible Bronchus-Associated Lymphoid Tissues (iBALT) Serve as Sites of B Cell Selection and Maturation Following Influenza Infection in Mice.Front Immunol. 2019 Mar 29;10:611. doi: 10.3389/fimmu.2019.00611. eCollection 2019. Front Immunol. 2019. PMID: 30984186 Free PMC article.
-
Friend or Foe: The Protective and Pathological Roles of Inducible Bronchus-Associated Lymphoid Tissue in Pulmonary Diseases.J Immunol. 2019 May 1;202(9):2519-2526. doi: 10.4049/jimmunol.1801135. J Immunol. 2019. PMID: 31010841 Free PMC article. Review.
Cited by
-
Animal Models of Cryptococcus neoformans in Identifying Immune Parameters Associated With Primary Infection and Reactivation of Latent Infection.Front Immunol. 2020 Sep 15;11:581750. doi: 10.3389/fimmu.2020.581750. eCollection 2020. Front Immunol. 2020. PMID: 33042164 Free PMC article. Review.
-
Age differences in clinical features and outcomes in patients with COVID-19, Jiangsu, China: a retrospective, multicentre cohort study.BMJ Open. 2020 Oct 5;10(10):e039887. doi: 10.1136/bmjopen-2020-039887. BMJ Open. 2020. PMID: 33020106 Free PMC article.
-
Mouse Models Reveal Role of T-Cytotoxic and T-Reg Cells in Immune Response to Influenza: Implications for Vaccine Design.Viruses. 2019 Jan 11;11(1):52. doi: 10.3390/v11010052. Viruses. 2019. PMID: 30641955 Free PMC article. Review.
-
Role of iBALT in Respiratory Immunity.Curr Top Microbiol Immunol. 2020;426:21-43. doi: 10.1007/82_2019_191. Curr Top Microbiol Immunol. 2020. PMID: 31974759 Free PMC article. Review.
-
Tissue-resident immunity in the lung: a first-line defense at the environmental interface.Semin Immunopathol. 2022 Nov;44(6):827-854. doi: 10.1007/s00281-022-00964-2. Epub 2022 Oct 28. Semin Immunopathol. 2022. PMID: 36305904 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

