Deubiquitinase Usp8 regulates α-synuclein clearance and modifies its toxicity in Lewy body disease
- PMID: 27444016
- PMCID: PMC4987833
- DOI: 10.1073/pnas.1523597113
Deubiquitinase Usp8 regulates α-synuclein clearance and modifies its toxicity in Lewy body disease
Abstract
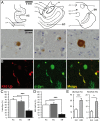
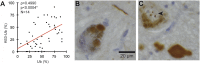
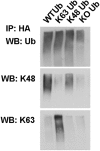
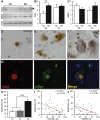
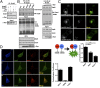
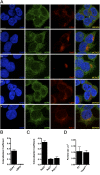
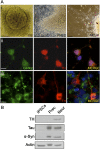
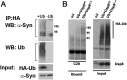
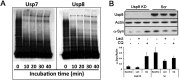
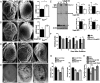
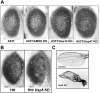
In Parkinson's disease, misfolded α-synuclein accumulates, often in a ubiquitinated form, in neuronal inclusions termed Lewy bodies. An important outstanding question is whether ubiquitination in Lewy bodies is directly relevant to α-synuclein trafficking or turnover and Parkinson's pathogenesis. By comparative analysis in human postmortem brains, we found that ubiquitin immunoreactivity in Lewy bodies is largely due to K63-linked ubiquitin chains and markedly reduced in the substantia nigra compared with the neocortex. The ubiquitin staining in cells with Lewy bodies inversely correlated with the content and pathological localization of the deubiquitinase Usp8. Usp8 interacted and partly colocalized with α-synuclein in endosomal membranes and, both in cells and after purification, it deubiquitinated K63-linked chains on α-synuclein. Knockdown of Usp8 in the Drosophila eye reduced α-synuclein levels and α-synuclein-induced eye toxicity. Accordingly, in human cells, Usp8 knockdown increased the lysosomal degradation of α-synuclein. In the dopaminergic neurons of the Drosophila model, unlike knockdown of other deubiquitinases, Usp8 protected from α-synuclein-induced locomotor deficits and cell loss. These findings strongly suggest that removal of K63-linked ubiquitin chains on α-synuclein by Usp8 is a critical mechanism that reduces its lysosomal degradation in dopaminergic neurons and may contribute to α-synuclein accumulation in Lewy body disease.
Keywords: Parkinson’s disease; endosome; neurodegeneration; ubiquitin; ubiquitin ligase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Ubiquitin ligase Nedd4 promotes alpha-synuclein degradation by the endosomal-lysosomal pathway.Proc Natl Acad Sci U S A. 2011 Oct 11;108(41):17004-9. doi: 10.1073/pnas.1109356108. Epub 2011 Sep 27. Proc Natl Acad Sci U S A. 2011. PMID: 21953697 Free PMC article.
-
Enhanced ubiquitin-dependent degradation by Nedd4 protects against α-synuclein accumulation and toxicity in animal models of Parkinson's disease.Neurobiol Dis. 2014 Apr;64(100):79-87. doi: 10.1016/j.nbd.2013.12.011. Epub 2013 Dec 31. Neurobiol Dis. 2014. PMID: 24388974 Free PMC article.
-
α-Synuclein fate is determined by USP9X-regulated monoubiquitination.Proc Natl Acad Sci U S A. 2011 Nov 15;108(46):18666-71. doi: 10.1073/pnas.1105725108. Epub 2011 Nov 7. Proc Natl Acad Sci U S A. 2011. PMID: 22065755 Free PMC article.
-
Ubiquitination of alpha-synuclein and autophagy in Parkinson's disease.Autophagy. 2008 Apr;4(3):372-4. doi: 10.4161/auto.5604. Epub 2008 Jan 18. Autophagy. 2008. PMID: 18216494 Review.
-
Is Cell Death Primary or Secondary in the Pathophysiology of Idiopathic Parkinson's Disease?Biomolecules. 2015 Jul 16;5(3):1467-79. doi: 10.3390/biom5031467. Biomolecules. 2015. PMID: 26193328 Free PMC article. Review.
Cited by
-
Protein modification in neurodegenerative diseases.MedComm (2020). 2024 Aug 4;5(8):e674. doi: 10.1002/mco2.674. eCollection 2024 Aug. MedComm (2020). 2024. PMID: 39105197 Free PMC article. Review.
-
USP19 deubiquitinase inactivation regulates α-synuclein ubiquitination and inhibits accumulation of Lewy body-like aggregates in mice.NPJ Parkinsons Dis. 2023 Nov 28;9(1):157. doi: 10.1038/s41531-023-00601-1. NPJ Parkinsons Dis. 2023. PMID: 38017009 Free PMC article.
-
Mitochondrial dysfunction and neurodegenerative proteinopathies: mechanisms and prospects for therapeutic intervention.Biochem Soc Trans. 2018 Aug 20;46(4):829-842. doi: 10.1042/BST20180025. Epub 2018 Jul 9. Biochem Soc Trans. 2018. PMID: 29986938 Free PMC article. Review.
-
Ubiquitin and Parkinson's disease through the looking glass of genetics.Biochem J. 2017 Apr 13;474(9):1439-1451. doi: 10.1042/BCJ20160498. Biochem J. 2017. PMID: 28408429 Free PMC article. Review.
-
A Critical Assessment of Exosomes in the Pathogenesis and Stratification of Parkinson's Disease.J Parkinsons Dis. 2017;7(4):569-576. doi: 10.3233/JPD-171176. J Parkinsons Dis. 2017. PMID: 28922170 Free PMC article. Review.
References
-
- Braak H, et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24(2):197–211. - PubMed
-
- Spillantini MG, et al. Alpha-synuclein in Lewy bodies. Nature. 1997;388(6645):839–840. - PubMed
-
- Wirdefeldt K, et al. Expression of alpha-synuclein in the human brain: Relation to Lewy body disease. Brain Res Mol Brain Res. 2001;92(1–2):58–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

