Antifungal activity of a β-peptide in synthetic urine media: Toward materials-based approaches to reducing catheter-associated urinary tract fungal infections
- PMID: 27422198
- PMCID: PMC5012908
- DOI: 10.1016/j.actbio.2016.07.016
Antifungal activity of a β-peptide in synthetic urine media: Toward materials-based approaches to reducing catheter-associated urinary tract fungal infections
Abstract
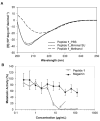
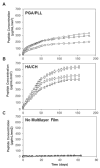
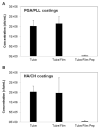
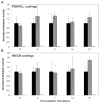
Catheter-associated urinary tract infections (CAUTI) are the most common type of hospital-acquired infection, with more than 30 million catheters placed annually in the US and a 10-30% incidence of infection. Candida albicans forms fungal biofilms on the surfaces of urinary catheters and is the leading cause of fungal urinary tract infections. As a step toward new strategies that could prevent or reduce the occurrence of C. albicans-based CAUTI, we investigated the ability of antifungal β-peptide-based mimetics of antimicrobial peptides (AMPs) to kill C. albicans and prevent biofilm formation in synthetic urine. Many α-peptide-based AMPs exhibit antifungal activities, but are unstable in high ionic strength media and are easily degraded by proteases-features that limit their use in urinary catheter applications. Here, we demonstrate that β-peptides designed to mimic the amphiphilic helical structures of AMPs retain 100% of their structural stability and exhibit antifungal and anti-biofilm activity against C. albicans in a synthetic medium that mimics the composition of urine. We demonstrate further that these agents can be loaded into and released from polymer-based multilayer coatings applied to polyurethane, polyethylene, and silicone tubing commonly used as urinary catheters. Our results reveal catheters coated with β-peptide-loaded multilayers to kill planktonic fungal cells for up to 21days of intermittent challenges with C. albicans and prevent biofilm formation on catheter walls for at least 48h. These new materials and approaches could lead to advances that reduce the occurrence of fungal CAUTI.
Statement of significance: Catheter-associated urinary tract infections are the most common type of hospital-acquired infection. The human pathogen Candida albicans is the leading cause of fungal urinary tract infections, and forms difficult to remove 'biofilms' on the surfaces of urinary catheters. We investigated synthetic β-peptide mimics of natural antimicrobial peptides as an approach to kill C. albicans and prevent biofilm formation in media that mimics the composition of urine. Our results reveal these mimics to retain structural stability and activity against C. albicans in synthetic urine. We also report polymer-based approaches to the local release of these agents within urinary catheter tubes. With further development, these materials-based approaches could lead to advances that reduce the occurrence of fungal urinary tract infections.
Keywords: Antifungal; Biofilms; Coatings; Multilayers; Urinary catheters; β-Peptides.
Copyright © 2016 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Figures




Similar articles
-
Polymer multilayers loaded with antifungal β-peptides kill planktonic Candida albicans and reduce formation of fungal biofilms on the surfaces of flexible catheter tubes.J Control Release. 2014 Oct 10;191:54-62. doi: 10.1016/j.jconrel.2014.05.026. Epub 2014 May 24. J Control Release. 2014. PMID: 24862322 Free PMC article.
-
Intraluminal Release of an Antifungal β-Peptide Enhances the Antifungal and Anti-Biofilm Activities of Multilayer-Coated Catheters in a Rat Model of Venous Catheter Infection.ACS Biomater Sci Eng. 2016 Jan 11;2(1):112-121. doi: 10.1021/acsbiomaterials.5b00427. Epub 2015 Dec 8. ACS Biomater Sci Eng. 2016. PMID: 26807439 Free PMC article.
-
Antimicrobial nanocomposite coatings for rapid intervention against catheter-associated urinary tract infections.Nanoscale. 2024 Jun 13;16(23):11109-11125. doi: 10.1039/d4nr00653d. Nanoscale. 2024. PMID: 38787647
-
Antimicrobial Peptides as a Strategy to Combat Fungal Biofilms.Curr Top Med Chem. 2017;17(5):604-612. doi: 10.2174/1568026616666160713142228. Curr Top Med Chem. 2017. PMID: 27411323 Review.
-
Antimicrobial strategies for urinary catheters.J Biomed Mater Res A. 2019 Feb;107(2):445-467. doi: 10.1002/jbm.a.36561. Epub 2018 Nov 23. J Biomed Mater Res A. 2019. PMID: 30468560 Review.
Cited by
-
Antimicrobial and antibiofilm activity of the EeCentrocin 1 derived peptide EC1-17KV via membrane disruption.EBioMedicine. 2020 May;55:102775. doi: 10.1016/j.ebiom.2020.102775. Epub 2020 May 11. EBioMedicine. 2020. PMID: 32403086 Free PMC article.
-
Synergic Effect of the Antimicrobial Peptide ToAP2 and Fluconazole on Candida albicans Biofilms.Int J Mol Sci. 2024 Jul 16;25(14):7769. doi: 10.3390/ijms25147769. Int J Mol Sci. 2024. PMID: 39063009 Free PMC article.
-
On-demand release of Candida albicans biofilms from urinary catheters by mechanical surface deformation.Biofouling. 2018 Jul;34(6):595-604. doi: 10.1080/08927014.2018.1474461. Epub 2018 Jun 13. Biofouling. 2018. PMID: 29897277 Free PMC article.
-
Recent Advances in Surface Nanoengineering for Biofilm Prevention and Control. Part II: Active, Combined Active and Passive, and Smart Bacteria-Responsive Antibiofilm Nanocoatings.Nanomaterials (Basel). 2020 Aug 4;10(8):1527. doi: 10.3390/nano10081527. Nanomaterials (Basel). 2020. PMID: 32759748 Free PMC article. Review.
-
Advances in Development of Antimicrobial Peptidomimetics as Potential Drugs.Molecules. 2017 Aug 29;22(9):1430. doi: 10.3390/molecules22091430. Molecules. 2017. PMID: 28850098 Free PMC article. Review.
References
-
- Siddiq DM, Darouiche RO. New strategies to prevent catheter-associated urinary tract infections. Nat Rev Urology. 2012;9(6):305–14. - PubMed
-
- Tambyah PA. Catheter-associated urinary tract infections: diagnosis and prophylaxis. Int J Antimicrob Ag. 2004;24:S44–S48. - PubMed
-
- Cardo D, Horan T, Andrus M, Dembinski M, Edwards J, Peavy G, Tolson J, Wagner D, Syst N. National Nosocomial Infections Surveillance (NNIS) system report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004;32(8):470–485. - PubMed
-
- Ramage G, Martinez JP, Lopez-Ribot JL. Candida biofilms on implanted biomaterials: a clinically significant problem. Fems Yeast Res. 2006;6(7):979–986. - PubMed
-
- Tambyah PA, Knasinski V, Maki DG. The direct costs of nosocomial catheter-associated urinary tract infection in the era of managed care. Infect Cont Hosp Ep. 2002;23(1):27–31. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

