Epicutaneous immunotherapy induces gastrointestinal LAP+ regulatory T cells and prevents food-induced anaphylaxis
- PMID: 27417020
- PMCID: PMC5149454
- DOI: 10.1016/j.jaci.2016.03.057
Epicutaneous immunotherapy induces gastrointestinal LAP+ regulatory T cells and prevents food-induced anaphylaxis
Abstract
Background: The attempt to induce oral tolerance as a treatment for food allergy has been hampered by a lack of sustained clinical protection. Immunotherapy by nonoral routes, such as the skin, may be more effective for the development of maintained tolerance to food allergens.
Objective: We sought to determine the efficacy and mechanism of tolerance induced by epicutaneous immunotherapy (EPIT) in a model of food-induced anaphylaxis.
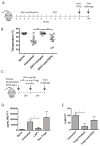
Methods: C3H/HeJ mice were sensitized to ovalbumin (OVA) orally or through the skin and treated with EPIT using OVA-Viaskin patches or oral immunotherapy using OVA. Mice were orally challenged with OVA to induce anaphylaxis. Antigen-specific regulatory T (Treg)-cell induction was assessed by flow cytometry using a transgenic T-cell transfer model.
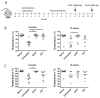
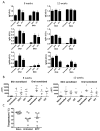
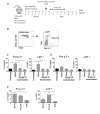
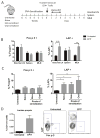
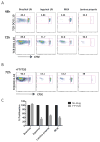
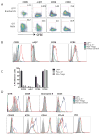
Results: By using an adjuvant-free model of food allergy generated by epicutaneous sensitization and reactions triggered by oral allergen challenge, we found that EPIT induced sustained protection against anaphylaxis. We show that the gastrointestinal tract is deficient in de novo generation of Treg cells in allergic mice. This defect was tissue-specific, and epicutaneous application of antigen generated a population of gastrointestinal-homing LAP+Foxp3- Treg cells. The mechanism of protection was found to be a novel pathway of direct TGF-β-dependent Treg-cell suppression of mast cell activation, in the absence of modulation of T- or B-cell responses.
Conclusions: Our data highlight the immune communication between skin and gastrointestinal tract, and identifies novel mechanisms by which epicutaneous tolerance can suppress food-induced anaphylaxis.
Keywords: Epicutaneous immunotherapy; food allergy; mast cells; oral immunotherapy; regulatory T cells.
Copyright © 2016 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosure of potential conflict of interest: LT received travel support from DBV Technologies (the developer and owner of the Viaskin® patch) to present this work in part at the European Academy of Allergy and Clinical Immunology. LM is an employee of DBV Technologies, HAS is the Chief Scientific Officer of DVB Technologies and PHB is CEO of DBV Technologies. MCB has no conflict of interest.
Figures
Similar articles
-
Antigen Uptake by Langerhans Cells Is Required for the Induction of Regulatory T Cells and the Acquisition of Tolerance During Epicutaneous Immunotherapy in OVA-Sensitized Mice.Front Immunol. 2018 Sep 3;9:1951. doi: 10.3389/fimmu.2018.01951. eCollection 2018. Front Immunol. 2018. PMID: 30233572 Free PMC article.
-
Specific epicutaneous immunotherapy prevents sensitization to new allergens in a murine model.J Allergy Clin Immunol. 2015 Jun;135(6):1546-57.e4. doi: 10.1016/j.jaci.2014.11.028. Epub 2015 Jan 9. J Allergy Clin Immunol. 2015. PMID: 25583102
-
The Role of Regulatory T Cells in Epicutaneous Immunotherapy for Food Allergy.Front Immunol. 2021 Jul 8;12:660974. doi: 10.3389/fimmu.2021.660974. eCollection 2021. Front Immunol. 2021. PMID: 34305893 Free PMC article. Review.
-
Laser-facilitated epicutaneous immunotherapy to IgE-mediated allergy.J Control Release. 2016 Aug 10;235:82-90. doi: 10.1016/j.jconrel.2016.05.057. Epub 2016 May 26. J Control Release. 2016. PMID: 27235977 Free PMC article.
-
The Current State of Epicutaneous Immunotherapy for Food Allergy: a Comprehensive Review.Clin Rev Allergy Immunol. 2018 Oct;55(2):153-161. doi: 10.1007/s12016-017-8650-3. Clin Rev Allergy Immunol. 2018. PMID: 29081025 Review.
Cited by
-
Therapeutic perspectives in food allergy.J Transl Med. 2020 Aug 5;18(1):302. doi: 10.1186/s12967-020-02466-x. J Transl Med. 2020. PMID: 32758254 Free PMC article. Review.
-
Evolution of Immune Responses in Food Immunotherapy.Immunol Allergy Clin North Am. 2020 Feb;40(1):87-95. doi: 10.1016/j.iac.2019.09.006. Epub 2019 Nov 6. Immunol Allergy Clin North Am. 2020. PMID: 31761123 Free PMC article. Review.
-
Mechanisms of Oral Tolerance.Clin Rev Allergy Immunol. 2018 Oct;55(2):107-117. doi: 10.1007/s12016-018-8680-5. Clin Rev Allergy Immunol. 2018. PMID: 29488131 Free PMC article. Review.
-
Genetic evidence for the role of transforming growth factor-β in atopic phenotypes.Curr Opin Immunol. 2019 Oct;60:54-62. doi: 10.1016/j.coi.2019.05.002. Epub 2019 Jun 1. Curr Opin Immunol. 2019. PMID: 31163387 Free PMC article. Review.
-
Deciphering the black box of food allergy mechanisms.Ann Allergy Asthma Immunol. 2017 Jan;118(1):21-27. doi: 10.1016/j.anai.2016.10.017. Ann Allergy Asthma Immunol. 2017. PMID: 28007085 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

