Shear-Induced Nitric Oxide Production by Endothelial Cells
- PMID: 27410748
- PMCID: PMC4944664
- DOI: 10.1016/j.bpj.2016.05.034
Shear-Induced Nitric Oxide Production by Endothelial Cells
Abstract
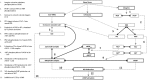
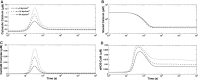
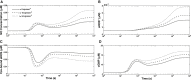
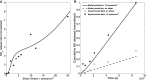
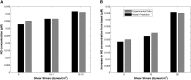
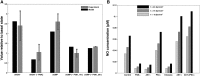
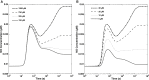
We present a biochemical model of the wall shear stress-induced activation of endothelial nitric oxide synthase (eNOS) in an endothelial cell. The model includes three key mechanotransducers: mechanosensing ion channels, integrins, and G protein-coupled receptors. The reaction cascade consists of two interconnected parts. The first is rapid activation of calcium, which results in formation of calcium-calmodulin complexes, followed by recruitment of eNOS from caveolae. The second is phosphorylation of eNOS by protein kinases PKC and AKT. The model also includes a negative feedback loop due to inhibition of calcium influx into the cell by cyclic guanosine monophosphate (cGMP). In this feedback, increased nitric oxide (NO) levels cause an increase in cGMP levels, so that cGMP inhibition of calcium influx can limit NO production. The model was used to predict the dynamics of NO production by an endothelial cell subjected to a step increase of wall shear stress from zero to a finite physiologically relevant value. Among several experimentally observed features, the model predicts a highly nonlinear, biphasic transient behavior of eNOS activation and NO production: a rapid initial activation due to the very rapid influx of calcium into the cytosol (occurring within 1-5 min) is followed by a sustained period of activation due to protein kinases.
Copyright © 2016 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Effects of pulsatile shear stress on signaling mechanisms controlling nitric oxide production, endothelial nitric oxide synthase phosphorylation, and expression in ovine fetoplacental artery endothelial cells.Endothelium. 2005 Jan-Apr;12(1-2):21-39. doi: 10.1080/10623320590933743. Endothelium. 2005. PMID: 16036314
-
AT1 receptor blocker potentiates shear-stress induced nitric oxide production via modulation of eNOS phosphorylation of residues Thr(495) and Ser(1177.).Biochem Biophys Res Commun. 2013 Nov 29;441(4):713-9. doi: 10.1016/j.bbrc.2013.10.108. Epub 2013 Nov 6. Biochem Biophys Res Commun. 2013. PMID: 24211212
-
Role of PECAM-1 in the shear-stress-induced activation of Akt and the endothelial nitric oxide synthase (eNOS) in endothelial cells.J Cell Sci. 2005 Sep 15;118(Pt 18):4103-11. doi: 10.1242/jcs.02541. Epub 2005 Aug 23. J Cell Sci. 2005. PMID: 16118242
-
Far-infrared radiation acutely increases nitric oxide production by increasing Ca(2+) mobilization and Ca(2+)/calmodulin-dependent protein kinase II-mediated phosphorylation of endothelial nitric oxide synthase at serine 1179.Biochem Biophys Res Commun. 2013 Jul 12;436(4):601-6. doi: 10.1016/j.bbrc.2013.06.003. Epub 2013 Jun 10. Biochem Biophys Res Commun. 2013. PMID: 23756809
-
Shear stress regulation of nitric oxide production in uterine and placental artery endothelial cells: experimental studies and hemodynamic models of shear stresses on endothelial cells.Int J Dev Biol. 2010;54(2-3):331-9. doi: 10.1387/ijdb.082832bs. Int J Dev Biol. 2010. PMID: 19876820 Free PMC article. Review.
Cited by
-
Differences in Blood Flow Patterns and Endothelial Shear Stress at the Carotid Artery Using Different Exercise Modalities and Intensities.Front Physiol. 2022 May 10;13:857816. doi: 10.3389/fphys.2022.857816. eCollection 2022. Front Physiol. 2022. PMID: 35620608 Free PMC article.
-
Yellow Nail Syndrome Successfully Treated with Oral Terbinafine and Topical Minoxidil.Clin Cosmet Investig Dermatol. 2021 Mar 12;14:249-252. doi: 10.2147/CCID.S301197. eCollection 2021. Clin Cosmet Investig Dermatol. 2021. PMID: 33746514 Free PMC article.
-
Deoxynivalenol induces structural alterations in epidermoid carcinoma cells A431 and impairs the response to biomechanical stimulation.Sci Rep. 2018 Jul 27;8(1):11351. doi: 10.1038/s41598-018-29728-5. Sci Rep. 2018. PMID: 30054545 Free PMC article.
-
The path to a hemocompatible cardiovascular implant: Advances and challenges of current endothelialization strategies.Front Cardiovasc Med. 2022 Sep 14;9:971028. doi: 10.3389/fcvm.2022.971028. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36186971 Free PMC article. Review.
-
The Role of Smoking in the Mechanisms of Development of Chronic Obstructive Pulmonary Disease and Atherosclerosis.Int J Mol Sci. 2023 May 13;24(10):8725. doi: 10.3390/ijms24108725. Int J Mol Sci. 2023. PMID: 37240069 Free PMC article. Review.
References
-
- Ignarro L.J. Endothelium-derived nitric oxide: actions and properties. FASEB J. 1989;3:31–36. - PubMed
-
- Wink D.A., Miranda K.M., Grisham M.B. Mechanisms of the antioxidant effects of nitric oxide. Antioxid. Redox Signal. 2001;3:203–213. - PubMed
-
- Balligand J.L., Feron O., Dessy C. eNOS activation by physical forces: from short-term regulation of contraction to chronic remodeling of cardiovascular tissues. Physiol. Rev. 2009;89:481–534. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

