On-Demand Dissolution of a Dendritic Hydrogel-based Dressing for Second-Degree Burn Wounds through Thiol-Thioester Exchange Reaction
- PMID: 27410669
- PMCID: PMC5168721
- DOI: 10.1002/anie.201604827
On-Demand Dissolution of a Dendritic Hydrogel-based Dressing for Second-Degree Burn Wounds through Thiol-Thioester Exchange Reaction
Abstract
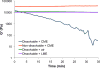
An adhesive yet easily removable burn wound dressing represents a breakthrough in second-degree burn wound care. Current second-degree burn wound dressings absorb wound exudate, reduce bacterial infections, and maintain a moist environment for healing, but are surgically or mechanically debrided from the wound, causing additional trauma to the newly formed tissues. We have developed an on-demand dissolvable dendritic thioester hydrogel burn dressing for second-degree burn care. The hydrogel is composed of a lysine-based dendron and a PEG-based crosslinker, which are synthesized in high yields. The hydrogel burn dressing covers the wound and acts as a barrier to bacterial infection in an in vivo second-degree burn wound model. A unique feature of the hydrogel is its capability to be dissolved on-demand, via a thiol-thioester exchange reaction, allowing for a facile burn dressing removal.
Keywords: dendrimers; dissolution; hydrogel; thiol-thioester exchange; wound dressing.
© 2016 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures


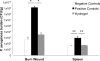
Similar articles
-
A dendritic thioester hydrogel based on thiol-thioester exchange as a dissolvable sealant system for wound closure.Angew Chem Int Ed Engl. 2013 Dec 23;52(52):14070-4. doi: 10.1002/anie.201308007. Epub 2013 Nov 26. Angew Chem Int Ed Engl. 2013. PMID: 24282150 Free PMC article.
-
On-Demand Dissolution of Chemically Cross-Linked Hydrogels.Acc Chem Res. 2017 Feb 21;50(2):151-160. doi: 10.1021/acs.accounts.6b00547. Epub 2017 Feb 7. Acc Chem Res. 2017. PMID: 28170219 Free PMC article.
-
Hyaluronate- and gelatin-based hydrogels encapsulating doxycycline as a wound dressing for burn injury therapy.Acta Biomater. 2023 Jul 1;164:151-158. doi: 10.1016/j.actbio.2023.04.021. Epub 2023 Apr 22. Acta Biomater. 2023. PMID: 37088160
-
Transparent Conductive Supramolecular Hydrogels with Stimuli-Responsive Properties for On-Demand Dissolvable Diabetic Foot Wound Dressings.Macromol Rapid Commun. 2020 Dec;41(24):e2000441. doi: 10.1002/marc.202000441. Epub 2020 Oct 21. Macromol Rapid Commun. 2020. PMID: 33089609 Review.
-
Functional Hydrogels as Wound Dressing to Enhance Wound Healing.ACS Nano. 2021 Aug 24;15(8):12687-12722. doi: 10.1021/acsnano.1c04206. Epub 2021 Aug 10. ACS Nano. 2021. PMID: 34374515 Review.
Cited by
-
Injectable and Self-Healing Chitosan Hydrogel Based on Imine Bonds: Design and Therapeutic Applications.Int J Mol Sci. 2018 Jul 27;19(8):2198. doi: 10.3390/ijms19082198. Int J Mol Sci. 2018. PMID: 30060504 Free PMC article. Review.
-
Dynamic Hydrogels with Viscoelasticity and Tunable Stiffness for the Regulation of Cell Behavior and Fate.Materials (Basel). 2023 Jul 21;16(14):5161. doi: 10.3390/ma16145161. Materials (Basel). 2023. PMID: 37512435 Free PMC article. Review.
-
Evaluation of Bacterial Cellulose Dressing versus Vaseline Gauze in Partial Thickness Burn Wounds and Skin Graft Donor Sites: A Two-Center Randomized Controlled Clinical Study.Evid Based Complement Alternat Med. 2022 May 18;2022:5217617. doi: 10.1155/2022/5217617. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 35656475 Free PMC article.
-
3D printing of sacrificial thioester elastomers using digital light processing for templating 3D organoid structures in soft biomatrices.Biofabrication. 2021 Sep 2;13(4):10.1088/1758-5090/ac1c98. doi: 10.1088/1758-5090/ac1c98. Biofabrication. 2021. PMID: 34380115 Free PMC article.
-
Theoretical-Experimental Approach of Chitosan/Quaternized Chitosan Nanofibers' Behavior in Wound Exudate Media.Pharmaceutics. 2023 Dec 2;15(12):2722. doi: 10.3390/pharmaceutics15122722. Pharmaceutics. 2023. PMID: 38140063 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

