Safety of histamine-2 receptor blockers in hospitalized VLBW infants
- PMID: 27390109
- PMCID: PMC4969147
- DOI: 10.1016/j.earlhumdev.2016.05.010
Safety of histamine-2 receptor blockers in hospitalized VLBW infants
Abstract
Background: Histamine-2 receptor (H2) blockers are often used in very low birth weight infants despite lack of population specific efficacy and safety data.
Aims: We sought to describe safety and temporal trends in histamine-2 receptor (H2) blocker use in hospitalized very low birth weight (VLBW) infants.
Study design: We conducted a retrospective cohort study using a clinical database populated by an electronic health record shared by 348 neonatal intensive care units in the United States.
Subjects: We included all VLBW infants without major congenital anomalies.
Outcome measures: We used multivariable logistic regression with generalizing estimating equations to evaluate the association between days of H2 blocker exposure and risk of: 1) death or necrotizing enterocolitis (NEC); 2) death or sepsis; and 3) death, NEC, or sepsis.
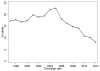
Results: Of 127,707 infants, 20,288 (16%) were exposed to H2 blockers for a total of 6,422,352days. Median gestational age for infants exposed to H2 blockers was 27weeks (25th 75th percentile 26, 29). H2 blocker use decreased from 18% of infants in 1997 to 8% in 2012 (p<0.001). On multivariable analysis, infants were at increased risk of the combined outcome of death, NEC, or sepsis on days exposed to H2 blockers (odds ratio=1.14) (95% confidence interval 1.08, 1.19).
Conclusions: H2 blocker use is associated with increased risk of the combined outcome of death, NEC, or sepsis in hospitalized VLBW infants.
Keywords: H2 blockers; Histamine-2 receptor antagonists; Infants; Necrotizing enterocolitis.
Copyright © 2016 Elsevier Ireland Ltd. All rights reserved.
Figures
Similar articles
-
Association Between Antibiotic Use and Neonatal Mortality and Morbidities in Very Low-Birth-Weight Infants Without Culture-Proven Sepsis or Necrotizing Enterocolitis.JAMA Pediatr. 2016 Dec 1;170(12):1181-1187. doi: 10.1001/jamapediatrics.2016.2132. JAMA Pediatr. 2016. PMID: 27775765
-
Exposure to Gastric Acid Inhibitors Increases the Risk of Infection in Preterm Very Low Birth Weight Infants but Concomitant Administration of Lactoferrin Counteracts This Effect.J Pediatr. 2018 Feb;193:62-67.e1. doi: 10.1016/j.jpeds.2017.09.080. Epub 2017 Dec 1. J Pediatr. 2018. PMID: 29198543 Clinical Trial.
-
Association of H2-blocker therapy and higher incidence of necrotizing enterocolitis in very low birth weight infants.Pediatrics. 2006 Feb;117(2):e137-42. doi: 10.1542/peds.2005-1543. Epub 2006 Jan 3. Pediatrics. 2006. PMID: 16390920
-
Association of inhibitors of gastric acid secretion and higher incidence of necrotizing enterocolitis in preterm very low-birth-weight infants.Am J Perinatol. 2013 Nov;30(10):849-56. doi: 10.1055/s-0033-1333671. Epub 2013 Jan 28. Am J Perinatol. 2013. PMID: 23359235 Review.
-
Slow advancement of enteral feed volumes to prevent necrotising enterocolitis in very low birth weight infants.Cochrane Database Syst Rev. 2017 Aug 30;8(8):CD001241. doi: 10.1002/14651858.CD001241.pub7. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2021 Aug 24;8:CD001241. doi: 10.1002/14651858.CD001241.pub8. PMID: 28854319 Free PMC article. Updated. Review.
Cited by
-
A Review of Histamine-2 Receptor Antagonist and Proton Pump Inhibitor Therapy for Gastroesophageal Reflux Disease in Neonates and Infants.Paediatr Drugs. 2023 Sep;25(5):557-576. doi: 10.1007/s40272-023-00580-z. Epub 2023 Jul 17. Paediatr Drugs. 2023. PMID: 37458926 Review.
-
Cost-effectiveness of probiotics for necrotizing enterocolitis prevention in very low birth weight infants.J Perinatol. 2020 Nov;40(11):1652-1661. doi: 10.1038/s41372-020-00790-0. Epub 2020 Aug 18. J Perinatol. 2020. PMID: 32811974
-
The challenges of neonatal sepsis management.J Pediatr (Rio J). 2020 Mar-Apr;96 Suppl 1(Suppl 1):80-86. doi: 10.1016/j.jped.2019.10.004. Epub 2019 Nov 17. J Pediatr (Rio J). 2020. PMID: 31747556 Free PMC article. Review.
-
Association between histamine-2 receptor antagonists and adverse outcomes in neonates: A systematic review and meta-analysis.PLoS One. 2019 Apr 4;14(4):e0214135. doi: 10.1371/journal.pone.0214135. eCollection 2019. PLoS One. 2019. PMID: 30947259 Free PMC article.
-
Neonatal intestinal dysbiosis in necrotizing enterocolitis.Mol Med. 2018 Mar 15;24(1):4. doi: 10.1186/s10020-018-0002-0. Mol Med. 2018. PMID: 30134786 Free PMC article. Review.
References
-
- van der Pol R, Langendam M, Benninga M, van Wijk M, Tabbers M. Efficacy and safety of histamine-2 receptor antagonists. JAMA Pediatr. 2014 Oct;168(10):947–54. - PubMed
-
- Malcolm WF, Cotten CM. Metoclopramide, H2 blockers, and proton pump inhibitors: pharmacotherapy for gastroesophageal reflux in neonates. Clin Perinatol. 2012 Mar;39(1):99–109. - PubMed
-
- Orenstein SR, Blumer JL, Faessel HM, McGuire JA, Fung K, Li BU, et al. Ranitidine, 75 mg, over-the-counter dose: pharmacokinetic and pharmacodynamic effects in children with symptoms of gastro-oesophageal reflux. Aliment Pharmacol Ther. 2002 May;16(5):899–907. - PubMed
-
- Oderda G, Dell'Olio D, Forni M, Farina L, Tavassoli K, Ansaldi N. Treatment of childhood peptic oesophagitis with famotidine or alginate-antacid. Ital J Gastroenterol. 1990 Dec;22(6):346–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources