Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy
- PMID: 27376576
- PMCID: PMC4935930
- DOI: 10.1038/nm.4123
Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy
Abstract
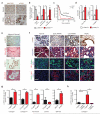
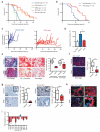
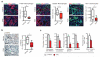
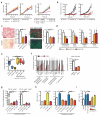
Single-agent immunotherapy has achieved limited clinical benefit to date in patients with pancreatic ductal adenocarcinoma (PDAC). This may be a result of the presence of a uniquely immunosuppressive tumor microenvironment (TME). Critical obstacles to immunotherapy in PDAC tumors include a high number of tumor-associated immunosuppressive cells and a uniquely desmoplastic stroma that functions as a barrier to T cell infiltration. We identified hyperactivated focal adhesion kinase (FAK) activity in neoplastic PDAC cells as an important regulator of the fibrotic and immunosuppressive TME. We found that FAK activity was elevated in human PDAC tissues and correlated with high levels of fibrosis and poor CD8(+) cytotoxic T cell infiltration. Single-agent FAK inhibition using the selective FAK inhibitor VS-4718 substantially limited tumor progression, resulting in a doubling of survival in the p48-Cre;LSL-Kras(G12D);Trp53(flox/+) (KPC) mouse model of human PDAC. This delay in tumor progression was associated with markedly reduced tumor fibrosis and decreased numbers of tumor-infiltrating immunosuppressive cells. We also found that FAK inhibition rendered the previously unresponsive KPC mouse model responsive to T cell immunotherapy and PD-1 antagonists. These data suggest that FAK inhibition increases immune surveillance by overcoming the fibrotic and immunosuppressive PDAC TME and renders tumors responsive to immunotherapy.
Figures

Comment in
-
Focal Adhesion Kinase: A promising therapeutic target in pancreatic adenocarcinoma.Clin Res Hepatol Gastroenterol. 2017 Jun;41(3):246-248. doi: 10.1016/j.clinre.2016.10.010. Epub 2016 Dec 6. Clin Res Hepatol Gastroenterol. 2017. PMID: 27939096
Similar articles
-
Exclusion of T Cells From Pancreatic Carcinomas in Mice Is Regulated by Ly6C(low) F4/80(+) Extratumoral Macrophages.Gastroenterology. 2015 Jul;149(1):201-10. doi: 10.1053/j.gastro.2015.04.010. Epub 2015 Apr 14. Gastroenterology. 2015. PMID: 25888329 Free PMC article.
-
Cadherin 11 Promotes Immunosuppression and Extracellular Matrix Deposition to Support Growth of Pancreatic Tumors and Resistance to Gemcitabine in Mice.Gastroenterology. 2021 Mar;160(4):1359-1372.e13. doi: 10.1053/j.gastro.2020.11.044. Epub 2020 Dec 9. Gastroenterology. 2021. PMID: 33307028 Free PMC article.
-
Development of resistance to FAK inhibition in pancreatic cancer is linked to stromal depletion.Gut. 2020 Jan;69(1):122-132. doi: 10.1136/gutjnl-2018-317424. Epub 2019 May 10. Gut. 2020. PMID: 31076405 Free PMC article.
-
Immune Checkpoint Inhibition for Pancreatic Ductal Adenocarcinoma: Current Limitations and Future Options.Front Immunol. 2018 Aug 15;9:1878. doi: 10.3389/fimmu.2018.01878. eCollection 2018. Front Immunol. 2018. PMID: 30158932 Free PMC article. Review.
-
Exploring the therapeutic potential of focal adhesion kinase inhibition in overcoming chemoresistance in pancreatic ductal adenocarcinoma.Future Med Chem. 2024 Feb;16(3):271-289. doi: 10.4155/fmc-2023-0234. Epub 2024 Jan 25. Future Med Chem. 2024. PMID: 38269431 Review.
Cited by
-
Pancreatic Adenocarcinoma Invasiveness and the Tumor Microenvironment: From Biology to Clinical Trials.Biomedicines. 2020 Oct 9;8(10):401. doi: 10.3390/biomedicines8100401. Biomedicines. 2020. PMID: 33050151 Free PMC article. Review.
-
Glycogen synthase kinase 3β: the nexus of chemoresistance, invasive capacity, and cancer stemness in pancreatic cancer.Cancer Drug Resist. 2024 Jan 31;7:4. doi: 10.20517/cdr.2023.84. eCollection 2024. Cancer Drug Resist. 2024. PMID: 38318525 Free PMC article. Review.
-
CTLA-4 in Regulatory T Cells for Cancer Immunotherapy.Cancers (Basel). 2021 Mar 22;13(6):1440. doi: 10.3390/cancers13061440. Cancers (Basel). 2021. PMID: 33809974 Free PMC article. Review.
-
Emerging roles for tumor stroma in antigen presentation and anti-cancer immunity.Biochem Soc Trans. 2023 Dec 20;51(6):2017-2028. doi: 10.1042/BST20221083. Biochem Soc Trans. 2023. PMID: 38031753 Free PMC article. Review.
-
The Relationship Between Dormant Cancer Cells and Their Microenvironment.Adv Cancer Res. 2016;132:45-71. doi: 10.1016/bs.acr.2016.07.002. Epub 2016 Aug 25. Adv Cancer Res. 2016. PMID: 27613129 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

