TNFα-activated mesenchymal stromal cells promote breast cancer metastasis by recruiting CXCR2+ neutrophils
- PMID: 27375023
- PMCID: PMC5290040
- DOI: 10.1038/onc.2016.217
TNFα-activated mesenchymal stromal cells promote breast cancer metastasis by recruiting CXCR2+ neutrophils
Abstract
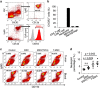
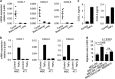
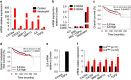
Mesenchymal stromal cells (MSCs) tend to infiltrate into tumors and form a major component of the tumor microenvironment. Our previous work demonstrated that tumor necrosis factor α (TNFα)-activated MSCs significantly promoted tumor growth. However, the role of TNFα-treated MSCs in tumor metastasis remains elusive. Employing a lung metastasis model of murine breast cancer, we found that TNFα-activated MSCs strikingly enhanced tumor metastasis compared with normal MSCs. We analyzed the chemokine profiles and found that the expression of CCL5, CCR2 and CXCR2 ligands were enhanced in TNFα-activated MSCs. Using genetic or pharmacological strategies to inhibit CCL5 or CCR2, we demonstrated that CCL5 and CCR2 ligands were indispensable in supporting TNFα-activated MSCs to promote tumor metastasis. Analysis of immune cells revealed that CXCR2 ligands (CXCL1, CXCL 2 and CXCL5) expressed by TNFα-activated MSCs efficiently recruited CXCR2+ neutrophils into tumor. These neutrophils were responsible for the pro-metastatic effect of MSCs since inhibition of this chemotaxis abolished increased neutrophil recruitment and tumor metastasis. The interaction between neutrophils and tumor cells resulted in markedly elevated metastasis-related genes by tumor cells, including CXCR4, CXCR7, MMP12, MMP13, IL-6 and TGFβ. Importantly, in IL8high human breast cancer samples, we also observed similar alterations of gene expression. Collectively, our findings demonstrate that TNFα-activated MSCs promote tumor metastasis via CXCR2+ neutrophil recruitment.
Figures



Similar articles
-
Regulation of the inflammatory profile of stromal cells in human breast cancer: prominent roles for TNF-α and the NF-κB pathway.Stem Cell Res Ther. 2015 May 1;6(1):87. doi: 10.1186/s13287-015-0080-7. Stem Cell Res Ther. 2015. PMID: 25928089 Free PMC article.
-
Downregulation of CXCL12 in mesenchymal stromal cells by TGFβ promotes breast cancer metastasis.Oncogene. 2017 Feb 9;36(6):840-849. doi: 10.1038/onc.2016.252. Epub 2016 Sep 26. Oncogene. 2017. PMID: 27669436 Free PMC article.
-
IL-17-CXC Chemokine Receptor 2 Axis Facilitates Breast Cancer Progression by Up-Regulating Neutrophil Recruitment.Am J Pathol. 2020 Jan;190(1):222-233. doi: 10.1016/j.ajpath.2019.09.016. Epub 2019 Oct 22. Am J Pathol. 2020. PMID: 31654638 Free PMC article.
-
CXCL5/CXCR2 axis in tumor microenvironment as potential diagnostic biomarker and therapeutic target.Cancer Commun (Lond). 2020 Mar;40(2-3):69-80. doi: 10.1002/cac2.12010. Cancer Commun (Lond). 2020. PMID: 32237072 Free PMC article. Review.
-
Recent Advances in Discovering the Role of CCL5 in Metastatic Breast Cancer.Mini Rev Med Chem. 2015;15(13):1063-72. doi: 10.2174/138955751513150923094709. Mini Rev Med Chem. 2015. PMID: 26420723 Free PMC article. Review.
Cited by
-
Adipose-derived mesenchymal stromal cells promote corneal wound healing by accelerating the clearance of neutrophils in cornea.Cell Death Dis. 2020 Aug 26;11(8):707. doi: 10.1038/s41419-020-02914-y. Cell Death Dis. 2020. PMID: 32848141 Free PMC article.
-
A neutrophil-biomimic platform for eradicating metastatic breast cancer stem-like cells by redox microenvironment modulation and hypoxia-triggered differentiation therapy.Acta Pharm Sin B. 2023 Jan;13(1):298-314. doi: 10.1016/j.apsb.2022.05.027. Epub 2022 May 29. Acta Pharm Sin B. 2023. PMID: 36815033 Free PMC article.
-
Immune modulation by mesenchymal stem cells.Cell Prolif. 2020 Jan;53(1):e12712. doi: 10.1111/cpr.12712. Epub 2019 Nov 15. Cell Prolif. 2020. PMID: 31730279 Free PMC article. Review.
-
The Safe and Efficacious Use of Secretome From Fibroblasts and Adipose-derived (but not Bone Marrow-derived) Mesenchymal Stem Cells for Skin Therapeutics.J Clin Aesthet Dermatol. 2019 Aug;12(8):E57-E69. Epub 2019 Aug 1. J Clin Aesthet Dermatol. 2019. PMID: 31531174 Free PMC article. Review.
-
Role of chemokine systems in cancer and inflammatory diseases.MedComm (2020). 2022 Jun 8;3(2):e147. doi: 10.1002/mco2.147. eCollection 2022 Jun. MedComm (2020). 2022. PMID: 35702353 Free PMC article. Review.
References
-
- Sasaki M, Abe R, Fujita Y, Ando S, Inokuma D, Shimizu H. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol 2008; 180: 2581–2587. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

