Pharmaceutical inhibition of mTOR in the common marmoset: effect of rapamycin on regulators of proteostasis in a non-human primate
- PMID: 27341957
- PMCID: PMC4920937
- DOI: 10.3402/pba.v6.31793
Pharmaceutical inhibition of mTOR in the common marmoset: effect of rapamycin on regulators of proteostasis in a non-human primate
Abstract
Background: Inhibition of mechanistic target of rapamycin (mTOR) has emerged as a viable means to lengthen lifespan and healthspan in mice, although it is still unclear whether these benefits will extend to other mammalian species. We previously reported results from a pilot experiment wherein common marmosets (Callithrix jacchus) were treated orally with rapamycin to reduce mTOR signaling in vivo in line with previous reports in mice and humans. Further, long-term treatment did not significantly alter body weight, daily activity, blood lipid concentrations, or glucose metabolism in this cohort.
Methods: In this study, we report on the molecular consequences of rapamycin treatment in marmosets on mechanisms that regulate protein homeostasis (proteostasis) in vivo. There is growing appreciation for the role of proteostasis in longevity and for the role that mTOR plays in regulating this process. Tissue samples of liver and skeletal muscle from marmosets in our pilot cohort were assessed for expression and activity of components of the ubiquitin-proteasome system, macroautophagy, and protein chaperones.
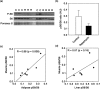
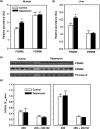
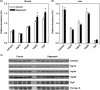
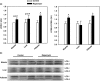
Results: Rapamycin treatment was associated with increased expression of PSMB5, a core subunit of the 20S proteasome, but not PSMB8 which is involved in the formation of the immunoproteasome, in the skeletal muscle and liver. Surprisingly, proteasome activity measured in these tissues was not affected by rapamycin. Rapamycin treatment was associated with an increased expression of mitochondria-targeted protein chaperones in skeletal muscle, but not liver. Finally, autophagy was increased in skeletal muscle and adipose, but not liver, from rapamycin-treated marmosets.
Conclusions: Overall, these data show tissue-specific upregulation of some, but not all, components of the proteostasis network in common marmosets treated with a pharmaceutical inhibitor of mTOR.
Keywords: autophagy; healthspan; immunoproteasome; proteasome; protein chaperone.
Figures
Similar articles
-
Metabolic consequences of long-term rapamycin exposure on common marmoset monkeys (Callithrix jacchus).Aging (Albany NY). 2015 Nov;7(11):964-73. doi: 10.18632/aging.100843. Aging (Albany NY). 2015. PMID: 26568298 Free PMC article.
-
Long-term treatment with the mTOR inhibitor rapamycin has minor effect on clinical laboratory markers in middle-aged marmosets.Am J Primatol. 2019 Feb;81(2):e22927. doi: 10.1002/ajp.22927. Epub 2018 Oct 12. Am J Primatol. 2019. PMID: 30311681 Free PMC article.
-
Testing efficacy of administration of the antiaging drug rapamycin in a nonhuman primate, the common marmoset.J Gerontol A Biol Sci Med Sci. 2015 May;70(5):577-87. doi: 10.1093/gerona/glu101. Epub 2014 Jul 19. J Gerontol A Biol Sci Med Sci. 2015. PMID: 25038772 Free PMC article.
-
Age-Related Dysfunction in Proteostasis and Cellular Quality Control in the Development of Sarcopenia.Cells. 2023 Jan 7;12(2):249. doi: 10.3390/cells12020249. Cells. 2023. PMID: 36672183 Free PMC article. Review.
-
Proteostasis and ageing: insights from long-lived mutant mice.J Physiol. 2017 Oct 15;595(20):6383-6390. doi: 10.1113/JP274334. Epub 2017 Aug 2. J Physiol. 2017. PMID: 28718225 Free PMC article. Review.
Cited by
-
Gerosuppression by pan-mTOR inhibitors.Aging (Albany NY). 2016 Dec 30;8(12):3535-3551. doi: 10.18632/aging.101155. Aging (Albany NY). 2016. PMID: 28077803 Free PMC article.
-
Nutrition, metabolism, and targeting aging in nonhuman primates.Ageing Res Rev. 2017 Oct;39:29-35. doi: 10.1016/j.arr.2017.02.002. Epub 2017 Feb 20. Ageing Res Rev. 2017. PMID: 28219777 Free PMC article. Review.
-
The development of a specific pathogen free (SPF) barrier colony of marmosets (Callithrix jacchus) for aging research.Aging (Albany NY). 2017 Dec 7;9(12):2544-2558. doi: 10.18632/aging.101340. Aging (Albany NY). 2017. PMID: 29227963 Free PMC article.
-
Roles of mitochondrial unfolded protein response in mammalian stem cells.World J Stem Cells. 2021 Jul 26;13(7):737-752. doi: 10.4252/wjsc.v13.i7.737. World J Stem Cells. 2021. PMID: 34367475 Free PMC article. Review.
-
Nutrition modulation of human aging: The calorie restriction paradigm.Mol Cell Endocrinol. 2017 Nov 5;455:148-157. doi: 10.1016/j.mce.2017.04.011. Epub 2017 Apr 12. Mol Cell Endocrinol. 2017. PMID: 28412520 Free PMC article. Review.
References
-
- Vellai T, Takacs-Vellai K, Zhang Y, Kovacs AL, Orosz L, Muller F. Genetics: influence of TOR kinase on lifespan in C. elegans. Nature. 2003;426(6967):620. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
