Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus
- PMID: 27339099
- PMCID: PMC4994874
- DOI: 10.1038/ni.3515
Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus
Abstract
Zika virus (ZIKV) was discovered in 1947 and was thought to lead to relatively mild disease. The recent explosive outbreak of ZIKV in South America has led to widespread concern, with reports of neurological sequelae ranging from Guillain Barré syndrome to microcephaly. ZIKV infection has occurred in areas previously exposed to dengue virus (DENV), a flavivirus closely related to ZIKV. Here we investigated the serological cross-reaction between the two viruses. Plasma immune to DENV showed substantial cross-reaction to ZIKV and was able to drive antibody-dependent enhancement (ADE) of ZIKV infection. Using a panel of human monoclonal antibodies (mAbs) to DENV, we showed that most antibodies that reacted to DENV envelope protein also reacted to ZIKV. Antibodies to linear epitopes, including the immunodominant fusion-loop epitope, were able to bind ZIKV but were unable to neutralize the virus and instead promoted ADE. Our data indicate that immunity to DENV might drive greater ZIKV replication and have clear implications for disease pathogenesis and future vaccine programs for ZIKV and DENV.
Conflict of interest statement
The EDE antibodies and epitope are the subject of a patent application by Imperial College and Institute Pasteur on which G.S., F.R., A.R. and G.B.S. are named as inventors
Figures

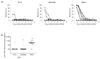
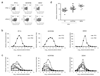
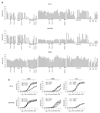


Comment in
-
Immunogenic cross-talk between dengue and Zika viruses.Nat Immunol. 2016 Aug 19;17(9):1010-2. doi: 10.1038/ni.3539. Nat Immunol. 2016. PMID: 27540984 No abstract available.
Similar articles
-
Humoral cross-reactivity between Zika and dengue viruses: implications for protection and pathology.Emerg Microbes Infect. 2017 May 10;6(5):e33. doi: 10.1038/emi.2017.42. Emerg Microbes Infect. 2017. PMID: 28487557 Free PMC article. Review.
-
Dengue Virus Envelope Dimer Epitope Monoclonal Antibodies Isolated from Dengue Patients Are Protective against Zika Virus.mBio. 2016 Jul 19;7(4):e01123-16. doi: 10.1128/mBio.01123-16. mBio. 2016. PMID: 27435464 Free PMC article.
-
Cross-reactive dengue virus-derived monoclonal antibodies to Zika virus envelope protein: Panacea or Pandora's box?BMC Infect Dis. 2018 Dec 10;18(1):641. doi: 10.1186/s12879-018-3572-0. BMC Infect Dis. 2018. PMID: 30526531 Free PMC article. Review.
-
A protective Zika virus E-dimer-based subunit vaccine engineered to abrogate antibody-dependent enhancement of dengue infection.Nat Immunol. 2019 Oct;20(10):1291-1298. doi: 10.1038/s41590-019-0477-z. Epub 2019 Sep 2. Nat Immunol. 2019. PMID: 31477918 Free PMC article.
-
Modulation of Dengue/Zika Virus Pathogenicity by Antibody-Dependent Enhancement and Strategies to Protect Against Enhancement in Zika Virus Infection.Front Immunol. 2018 Apr 23;9:597. doi: 10.3389/fimmu.2018.00597. eCollection 2018. Front Immunol. 2018. PMID: 29740424 Free PMC article. Review.
Cited by
-
Zika Fetal Neuropathogenesis: Etiology of a Viral Syndrome.PLoS Negl Trop Dis. 2016 Aug 25;10(8):e0004877. doi: 10.1371/journal.pntd.0004877. eCollection 2016 Aug. PLoS Negl Trop Dis. 2016. PMID: 27560129 Free PMC article. Review.
-
Plant-expressed Zika virus envelope protein elicited protective immunity against the Zika virus in immunocompetent mice.Sci Rep. 2023 Dec 27;13(1):22955. doi: 10.1038/s41598-023-47428-7. Sci Rep. 2023. PMID: 38151523 Free PMC article.
-
Serological Evidence of Zika Virus Infections in Sudan.Viruses. 2024 Jun 28;16(7):1045. doi: 10.3390/v16071045. Viruses. 2024. PMID: 39066208 Free PMC article.
-
Prior flavivirus immunity skews the yellow fever vaccine response to cross-reactive antibodies with potential to enhance dengue virus infection.Nat Commun. 2024 Feb 24;15(1):1696. doi: 10.1038/s41467-024-45806-x. Nat Commun. 2024. PMID: 38402207 Free PMC article.
-
B-Cell Memory Responses to Variant Viral Antigens.Viruses. 2021 Mar 26;13(4):565. doi: 10.3390/v13040565. Viruses. 2021. PMID: 33810456 Free PMC article. Review.
References
-
- World Health Organization. Zika Virus Fact sheet. 2016 http://www.who.int/mediacentre/factsheets/zika/en/
-
- Duffy MR, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360:2536–2543. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

