Retromer in Osteoblasts Interacts With Protein Phosphatase 1 Regulator Subunit 14C, Terminates Parathyroid Hormone's Signaling, and Promotes Its Catabolic Response
- PMID: 27333042
- PMCID: PMC4972523
- DOI: 10.1016/j.ebiom.2016.05.028
Retromer in Osteoblasts Interacts With Protein Phosphatase 1 Regulator Subunit 14C, Terminates Parathyroid Hormone's Signaling, and Promotes Its Catabolic Response
Abstract
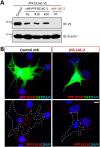
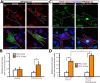
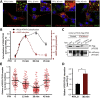
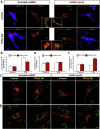
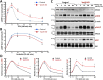
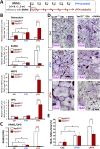
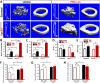
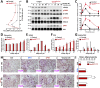
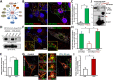
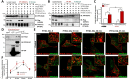
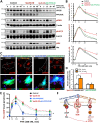
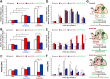
Parathyroid hormone (PTH) plays critical, but distinct, roles in bone remodeling, including bone formation (anabolic response) and resorption (catabolic response). Although its signaling and function have been extensively investigated, it just began to be understood how distinct functions are induced by PTH activating a common receptor, the PTH type 1 receptor (PTH1R), and how PTH1R signaling is terminated. Here, we provide evidence for vacuolar protein sorting 35 (VPS35), a major component of retromer, in regulating PTH1R trafficking, turning off PTH signaling, and promoting its catabolic function. VPS35 is expressed in osteoblast (OB)-lineage cells. VPS35-deficiency in OBs impaired PTH(1-34)-promoted PTH1R translocation to the trans-Golgi network, enhanced PTH(1-34)-driven signaling, and reduced PTH(1-34)'s catabolic response in culture and in mice. Further mechanical studies revealed that VPS35 interacts with not only PTH1R, but also protein phosphatase 1 regulatory subunit 14C (PPP1R14C), an inhibitory subunit of PP1 phosphatase. PPP1R14C also interacts with PTH1R, which is necessary for the increased endosomal PTH1R signaling and decreased PTH(1-34)'s catabolic response in VPS35-deficient OB-lineage cells. Taken together, these results suggest that VPS35 deregulates PTH1R-signaling likely by its interaction with PTH1R and PPP1R14C. This event is critical for the control of PTH(1-34)-signaling dynamics, which may underlie PTH-induced catabolic response and adequate bone remodeling.
Keywords: Osteoblasts; PPP1R14C; PTH1R; Retromer; VPS35.
Published by Elsevier B.V.
Figures


Similar articles
-
VPS35: Two Ways to Recycle the Parathyroid Hormone Receptor (PTH1R) in Osteoblasts.EBioMedicine. 2016 Jul;9:3-4. doi: 10.1016/j.ebiom.2016.06.029. Epub 2016 Jun 23. EBioMedicine. 2016. PMID: 27374314 Free PMC article. No abstract available.
-
Control of Bone Anabolism in Response to Mechanical Loading and PTH by Distinct Mechanisms Downstream of the PTH Receptor.J Bone Miner Res. 2017 Mar;32(3):522-535. doi: 10.1002/jbmr.3011. Epub 2016 Oct 27. J Bone Miner Res. 2017. PMID: 27704638 Free PMC article.
-
TGF-beta type II receptor phosphorylates PTH receptor to integrate bone remodelling signalling.Nat Cell Biol. 2010 Mar;12(3):224-34. doi: 10.1038/ncb2022. Epub 2010 Feb 7. Nat Cell Biol. 2010. PMID: 20139972 Free PMC article.
-
Arrestins in bone.Prog Mol Biol Transl Sci. 2013;118:335-58. doi: 10.1016/B978-0-12-394440-5.00013-9. Prog Mol Biol Transl Sci. 2013. PMID: 23764060 Review.
-
Biased agonism at the parathyroid hormone receptor: a demonstration of functional selectivity in bone metabolism.Mini Rev Med Chem. 2012 Aug;12(9):856-65. doi: 10.2174/138955712800959125. Mini Rev Med Chem. 2012. PMID: 22681253 Review.
Cited by
-
Differential Expression of MED12-Associated Coding RNA Transcripts in Uterine Leiomyomas.Int J Mol Sci. 2023 Feb 13;24(4):3742. doi: 10.3390/ijms24043742. Int J Mol Sci. 2023. PMID: 36835153 Free PMC article.
-
Linking skeletal muscle aging with osteoporosis by lamin A/C deficiency.PLoS Biol. 2020 Jun 1;18(6):e3000731. doi: 10.1371/journal.pbio.3000731. eCollection 2020 Jun. PLoS Biol. 2020. PMID: 32479501 Free PMC article.
-
Anabolic actions of PTH in murine models: two decades of insights.J Bone Miner Res. 2021 Oct;36(10):1979-1998. doi: 10.1002/jbmr.4389. Epub 2021 Jul 27. J Bone Miner Res. 2021. PMID: 34101904 Free PMC article.
-
Updated Insight into the Physiological and Pathological Roles of the Retromer Complex.Int J Mol Sci. 2017 Jul 25;18(8):1601. doi: 10.3390/ijms18081601. Int J Mol Sci. 2017. PMID: 28757549 Free PMC article. Review.
-
Identification of potential pathogenic genes associated with osteoporosis.Bone Joint Res. 2017 Dec;6(12):640-648. doi: 10.1302/2046-3758.612.BJR-2017-0102.R1. Bone Joint Res. 2017. PMID: 29203636 Free PMC article.
References
-
- Belenkaya T.Y., Wu Y., Tang X., Zhou B., Cheng L., Sharma Y.V., Yan D., Selva E.M., Lin X. The retromer complex influences Wnt secretion by recycling wntless from endosomes to the trans-Golgi network. Dev. Cell. 2008;14:120–131. - PubMed
-
- Borroni B., Ferrari F., Galimberti D., Nacmias B., Barone C., Bagnoli S., Fenoglio C., Piaceri I., Archetti S., Bonvicini C. Heterozygous TREM2 mutations in frontotemporal dementia. Neurobiol. Aging. 2014;35(934) (e937-910) - PubMed
-
- Chen D., Xiao H., Zhang K., Wang B., Gao Z., Jian Y., Qi X., Sun J., Miao L., Yang C. Retromer is required for apoptotic cell clearance by phagocytic receptor recycling. Science. 2010;327:1261–1264. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

