Tumor-associated fibroblasts predominantly come from local and not circulating precursors
- PMID: 27317748
- PMCID: PMC4941507
- DOI: 10.1073/pnas.1600363113
Tumor-associated fibroblasts predominantly come from local and not circulating precursors
Abstract
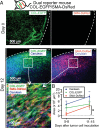
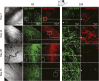
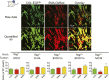
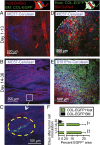
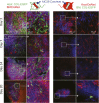
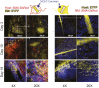
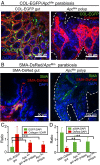
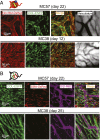
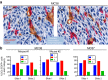
Fibroblasts are common cell types in cancer stroma and lay down collagen required for survival and growth of cancer cells. Although some cancer therapy strategies target tumor fibroblasts, their origin remains controversial. Multiple publications suggest circulating mesenchymal precursors as a source of tumor-associated fibroblasts. However, we show by three independent approaches that tumor fibroblasts derive primarily from local, sessile precursors. First, transplantable tumors developing in a mouse expressing green fluorescent reporter protein (EGFP) under control of the type I collagen (Col-I) promoter (COL-EGFP) had green stroma, whereas we could not find COL-EGFP(+) cells in tumors developing in the parabiotic partner lacking the fluorescent reporter. Lack of incorporation of COL-EGFP(+) cells from the circulation into tumors was confirmed in parabiotic pairs of COL-EGFP mice and transgenic mice developing autochthonous intestinal adenomas. Second, transplantable tumors developing in chimeric mice reconstituted with bone marrow cells from COL-EGFP mice very rarely showed stromal fibroblasts expressing EGFP. Finally, cancer cells injected under full-thickness COL-EGFP skin grafts transplanted in nonreporter mice developed into tumors containing green stromal cells. Using multicolor in vivo confocal microscopy, we found that Col-I-expressing fibroblasts constituted approximately one-third of the stromal mass and formed a continuous sheet wrapping the tumor vessels. In summary, tumors form their fibroblastic stroma predominantly from precursors present in the local tumor microenvironment, whereas the contribution of bone marrow-derived circulating precursors is rare.
Keywords: bone marrow; collagen; mesenchymal; origin; stroma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Hematopoietic origins of fibroblasts: I. In vivo studies of fibroblasts associated with solid tumors.Exp Hematol. 2006 Feb;34(2):208-18. doi: 10.1016/j.exphem.2005.10.009. Exp Hematol. 2006. PMID: 16459189
-
Differential contribution of dermal resident and bone marrow-derived cells to collagen production during wound healing and fibrogenesis in mice.J Invest Dermatol. 2011 Feb;131(2):529-36. doi: 10.1038/jid.2010.314. Epub 2010 Oct 21. J Invest Dermatol. 2011. PMID: 20962852
-
Contribution of bone marrow-derived cells to skin: collagen deposition and wound repair.Stem Cells. 2004;22(5):812-22. doi: 10.1634/stemcells.22-5-812. Stem Cells. 2004. PMID: 15342945 Free PMC article.
-
The cyan fluorescent protein nude mouse as a host for multicolor-coded imaging models of primary and metastatic tumor microenvironments.Anticancer Res. 2012 Jan;32(1):31-8. Anticancer Res. 2012. PMID: 22213285
-
Tumor-stroma interactions directing phenotype and progression of epithelial skin tumor cells.Differentiation. 2002 Dec;70(9-10):486-97. doi: 10.1046/j.1432-0436.2002.700903.x. Differentiation. 2002. PMID: 12492491 Review.
Cited by
-
TGFβ1 in Cancer-Associated Fibroblasts Is Associated With Progression and Radiosensitivity in Small-Cell Lung Cancer.Front Cell Dev Biol. 2021 May 20;9:667645. doi: 10.3389/fcell.2021.667645. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34095135 Free PMC article.
-
Microenvironmental modulation of the developing tumour: an immune-stromal dialogue.Mol Oncol. 2021 Oct;15(10):2600-2633. doi: 10.1002/1878-0261.12773. Epub 2020 Aug 28. Mol Oncol. 2021. PMID: 32741067 Free PMC article. Review.
-
Signaling pathways in cancer-associated fibroblasts: recent advances and future perspectives.Cancer Commun (Lond). 2023 Jan;43(1):3-41. doi: 10.1002/cac2.12392. Epub 2022 Nov 24. Cancer Commun (Lond). 2023. PMID: 36424360 Free PMC article. Review.
-
Define cancer-associated fibroblasts (CAFs) in the tumor microenvironment: new opportunities in cancer immunotherapy and advances in clinical trials.Mol Cancer. 2023 Oct 2;22(1):159. doi: 10.1186/s12943-023-01860-5. Mol Cancer. 2023. PMID: 37784082 Free PMC article. Review.
-
A framework for advancing our understanding of cancer-associated fibroblasts.Nat Rev Cancer. 2020 Mar;20(3):174-186. doi: 10.1038/s41568-019-0238-1. Epub 2020 Jan 24. Nat Rev Cancer. 2020. PMID: 31980749 Free PMC article. Review.
References
-
- Metchnikoff E. Leçons sur la pathologie comparée de l’inflammation. Faites à l’Institut Pasteur en Avril et Mai 1891. Masson; Paris: 1892.
-
- Maximov A. Culture of blood leukocytes. From lymphocytes and monocytes to connective tissue. Arch Exp Zellforsch. 1928;5:169–268.
-
- Friedenstein A. Stromal-hematopoietic interrelationships: Maximov’s ideas and modern models. Haematol Blood Transfus. 1989;32:159–167. - PubMed
-
- Labat ML, et al. Cystic fibrosis: Production of high levels of uromodulin-like protein by HLA-DR blood monocytes differentiating towards a fibroblastic phenotype. Biomed Pharmacother. 1991;45(9):387–401. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

