Lenalidomide, Thalidomide, and Pomalidomide Reactivate the Epstein-Barr Virus Lytic Cycle through Phosphoinositide 3-Kinase Signaling and Ikaros Expression
- PMID: 27297582
- PMCID: PMC5050094
- DOI: 10.1158/1078-0432.CCR-15-2242
Lenalidomide, Thalidomide, and Pomalidomide Reactivate the Epstein-Barr Virus Lytic Cycle through Phosphoinositide 3-Kinase Signaling and Ikaros Expression
Abstract
Purpose: Lenalidomide, thalidomide, and pomalidomide (LTP) are immunomodulatory agents approved for use in multiple myeloma, but in some settings, especially with alkylating agents, an increase in Hodgkin lymphoma and other secondary primary malignancies (SPM) has been noted. Some of these malignancies have been linked to Epstein-Barr virus (EBV), raising the possibility that immunomodulatory drugs disrupt latent EBV infection.
Experimental design: We studied the ability of LTP to reactivate latently infected EBV-positive cell lines in vitro and in vivo, and evaluated the EBV viral load in archived serum samples from patients who received a lenalidomide, thalidomide, and dexamethasone (LTD) combination.
Results: Treatment of EBV-infected B-cell lines with LTP at physiologically relevant concentrations induced the immediate early gene BZLF1, the early gene BMRF1, and the late proteins VCA and BCFR1. This occurred in the potency order pomalidomide > lenalidomide > thalidomide, and the nucleoside analogue ganciclovir enhanced the cytotoxic effects of lenalidomide and pomalidomide in Burkitt lymphoma cells in vitro and in vivo EBV reactivation was related to PI3K stimulation and Ikaros suppression, and blocked by the PI3Kδ inhibitor idelalisib. Combinations of lenalidomide with dexamethasone or rituximab increased EBV reactivation compared with lenalidomide alone and, importantly, lenalidomide with melphalan produced even greater reactivation.
Conclusions: We conclude LTP may reactivate EBV-positive resting memory B cells thereby enhancing EBV lytic cycle and host immune suppression. Clin Cancer Res; 22(19); 4901-12. ©2016 AACR.
©2016 American Association for Cancer Research.
Conflict of interest statement
The remaining authors declare no financial conflicts of interest.
Figures

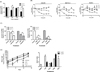
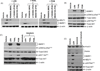
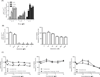


Similar articles
-
Inhibition of Epstein-Barr Virus Lytic Reactivation by the Atypical Antipsychotic Drug Clozapine.Viruses. 2019 May 17;11(5):450. doi: 10.3390/v11050450. Viruses. 2019. PMID: 31108875 Free PMC article.
-
Lytic induction therapy for Epstein-Barr virus-positive B-cell lymphomas.J Virol. 2004 Feb;78(4):1893-902. doi: 10.1128/jvi.78.4.1893-1902.2004. J Virol. 2004. PMID: 14747554 Free PMC article.
-
The B-cell specific transcription factor, Oct-2, promotes Epstein-Barr virus latency by inhibiting the viral immediate-early protein, BZLF1.PLoS Pathog. 2012 Feb;8(2):e1002516. doi: 10.1371/journal.ppat.1002516. Epub 2012 Feb 9. PLoS Pathog. 2012. PMID: 22346751 Free PMC article.
-
[Pomalidomide in the treatment of relapsed and refractory multiple myeloma].Klin Onkol. 2014;27(5):318-25. doi: 10.14735/amko2014318. Klin Onkol. 2014. PMID: 25312708 Review. Czech.
-
Epstein--Barr virus post-transplant lymphoproliferative disease and virus-specific therapy: pharmacological re-activation of viral target genes with arginine butyrate.Transpl Infect Dis. 2001 Sep;3(3):177-85. doi: 10.1034/j.1399-3062.2001.003003177.x. Transpl Infect Dis. 2001. PMID: 11493400 Review.
Cited by
-
Epigenetic crossroads of the Epstein-Barr virus B-cell relationship.Curr Opin Virol. 2018 Oct;32:15-23. doi: 10.1016/j.coviro.2018.08.012. Epub 2018 Sep 15. Curr Opin Virol. 2018. PMID: 30227386 Free PMC article. Review.
-
Management of post-transplant lymphoproliferative disorders.Hemasphere. 2019 Jun 30;3(Suppl):74-77. doi: 10.1097/HS9.0000000000000226. eCollection 2019 Jun. Hemasphere. 2019. PMID: 35309814 Free PMC article. No abstract available.
-
A new therapy in Epstein-Barr virus-associated lymphoproliferative disease: a case report and a revision of the literature.Ital J Pediatr. 2019 Nov 4;45(1):135. doi: 10.1186/s13052-019-0741-8. Ital J Pediatr. 2019. PMID: 31685000 Free PMC article. Review.
-
Acute Lymphoblastic Leukemia following Lenalidomide Maintenance for Multiple Myeloma: Two Cases with Unexpected Presentation and Good Prognostic Features.Case Rep Hematol. 2018 Feb 12;2018:9052314. doi: 10.1155/2018/9052314. eCollection 2018. Case Rep Hematol. 2018. PMID: 29785311 Free PMC article.
-
Biological Difference Between Epstein-Barr Virus Positive and Negative Post-transplant Lymphoproliferative Disorders and Their Clinical Impact.Front Oncol. 2020 May 8;10:506. doi: 10.3389/fonc.2020.00506. eCollection 2020. Front Oncol. 2020. PMID: 32457824 Free PMC article. Review.
References
-
- Jagannath S. Introduction: addressing challenges in multiple myeloma management in an era of new therapeutics. Journal of the National Comprehensive Cancer Network : JNCCN. 2010;8(Suppl 1):S1–S3. - PubMed
-
- Attal M, Lauwers-Cances V, Marit G, Caillot D, Moreau P, Facon T, et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. The New England journal of medicine. 2012;366:1782–1791. - PubMed
-
- Palumbo A, Hajek R, Delforge M, Kropff M, Petrucci MT, Catalano J, et al. Continuous lenalidomide treatment for newly diagnosed multiple myeloma. The New England journal of medicine. 2012;366:1759–1769. - PubMed
-
- Dimopoulos MA, Richardson PG, Brandenburg N, Yu Z, Weber DM, Niesvizky R, et al. A review of second primary malignancy in patients with relapsed or refractory multiple myeloma treated with lenalidomide. Blood. 2012;119:2764–2767. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

