Immune checkpoint blockade with concurrent electrochemotherapy in advanced melanoma: a retrospective multicenter analysis
- PMID: 27294607
- PMCID: PMC11029138
- DOI: 10.1007/s00262-016-1856-z
Immune checkpoint blockade with concurrent electrochemotherapy in advanced melanoma: a retrospective multicenter analysis
Abstract
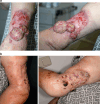
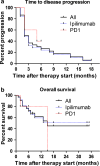
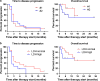
Growing evidence suggests that concurrent loco-regional and systemic treatment modalities may lead to synergistic anti-tumor effects in advanced melanoma. In this retrospective multicenter study, we evaluate the use of electrochemotherapy (ECT) combined with ipilimumab or PD-1 inhibition. We investigated patients with unresectable or metastatic melanoma who received the combination of ECT and immune checkpoint blockade for distant or cutaneous metastases within 4 weeks. Clinical and laboratory data were collected and analyzed with respect to safety and efficacy. A total of 33 patients from 13 centers were identified with a median follow-up time of 9 months. Twenty-eight patients received ipilimumab, while five patients were treated with a PD-1 inhibitor (pembrolizumab n = 3, nivolumab n = 2). The local overall response rate (ORR) was 66.7 %. The systemic ORR was 19.2 and 40.0 % in the ipilimumab and PD-1 cohort, respectively. The median duration of response was not reached in either group. The median time to disease progression was 2.5 months for the entire population with 2 months for ipilimumab and 5 months for PD-1 blockade. The median overall survival was not reached in patients with ipilimumab and 15 months in the PD-1 group. Severe systemic adverse events were detected in 25.0 % in the ipilimumab group. No treatment-related deaths were observed. This is the first reported evaluation of ECT and simultaneous PD-1 inhibition and the largest published dataset on ECT with concurrent ipilimumab. The local response was lower than reported for ECT only. Ipilimumab combined with ECT was feasible, tolerable and showed a high systemic response rate.
Keywords: Electrochemotherapy; Immune checkpoint blockade; Ipilimumab; Melanoma; Nivolumab; Pembrolizumab.
Conflict of interest statement
Beatrice Schell, Edgar Dippel, Fanny Matheis, Markus V. Heppt, Susanne G. Schäd, and Thilo Gambichler declare no conflict of interest. Bastian Schilling: advisory for Roche, M.S.D. Sharp and Dohme, and Bristol-Myers Squibb, travel support from Roche, Bristol-Myers Squibb and Amgen, honoraria from Roche, M.S.D. Sharp and Dohme and Bristol-Myers Squibb. Carola Berking: advisory for Amgen, AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, M.S.D. Sharp and Dohme, Novartis, Roche, speaker’s honoraria by Bristol-Myers Squibb, GlaxoSmithKline, M.S.D. Sharp and Dohme, Novartis, Roche. Carmen Loquai: advisory for Roche, Amgen, Novartis, Bristol-Myers Squibb, M.S.D. Sharp and Dohme, Ribological, speaker’s honoraria from Roche, Bristol-Myers Squibb, M.S.D. Sharp and Dohme, Novartis, travel reimbursement from Roche, Bristol-Myers Squibb, M.S.D. Sharp and Dohme, Novartis. Daniela Göppner: advisory for Roche, Amgen, Bristol-Myers Squibb and M.S.D. Sharp and Dohme, speaker’s honoraria from Roche and Bristol-Myers Squibb, travel reimbursement from Roche, Amgen and Novartis. Erwin S. Schultz: advisory for Bristol-Myers Squibb and Novartis, speaker’s honoraria for Novartis. Julia K. Tietze: speaker’s honoraria from Bristol-Myers Squibb, M.S.D. Sharp and Dohme, Novartis, Roche. Jens Ulrich: advisory for Roche, speaker’s honoraria from Bristol-Myers Squibb, M.S.D. Sharp and Dohme, GlaxoSmithKline, IGEA, Novartis, Roche, travel reimbursement from Bristol-Myers Squibb, IGEA, Medac, Roche. Katharina C. Kähler: advisory for Roche, Amgen, Bristol-Myers Squibb, M.S.D. Sharp and Dohme, speaker’s honoraria from Roche, Bristol-Myers Squibb, M.S.D. Sharp and Dohme, Novartis, travel reimbursement from Roche, Bristol-Myers Squibb, M.S.D. Sharp and Dohme, Novartis. Rudolf A. Herbst: advisory for Amgen, Bristol-Myers Squibb, GlaxoSmithKline, Novartis, Roche, speaker’s honoraria from Bristol-Myers Squibb, GlaxoSmithKline, M.S.D. Sharp and Dohme, Novartis, Roche. Thomas K. Eigentler: advisory for AMGEN, Roche, Bristol-Myers Squibb, travel support from Bristol-Myers Squibb and Novartis, speaker’s honoraria from Roche.
Figures
Similar articles
-
Ipilimumab alone or ipilimumab plus anti-PD-1 therapy in patients with metastatic melanoma resistant to anti-PD-(L)1 monotherapy: a multicentre, retrospective, cohort study.Lancet Oncol. 2021 Jun;22(6):836-847. doi: 10.1016/S1470-2045(21)00097-8. Epub 2021 May 11. Lancet Oncol. 2021. PMID: 33989557
-
Systematic review and meta-analysis efficacy and safety of immune checkpoint inhibitors in advanced melanoma patients with anti-PD-1 progression: a systematic review and meta-analysis.Clin Transl Oncol. 2021 Sep;23(9):1885-1904. doi: 10.1007/s12094-021-02598-6. Epub 2021 Apr 20. Clin Transl Oncol. 2021. PMID: 33877531
-
MAPKinase inhibition after failure of immune checkpoint blockade in patients with advanced melanoma - An evaluation of the multicenter prospective skin cancer registry ADOREG.Eur J Cancer. 2022 May;167:32-41. doi: 10.1016/j.ejca.2022.02.023. Epub 2022 Mar 30. Eur J Cancer. 2022. PMID: 35366571
-
Unmasking of intracranial metastatic melanoma during ipilimumab/nivolumab therapy: case report and literature review.BMC Cancer. 2018 May 9;18(1):549. doi: 10.1186/s12885-018-4470-y. BMC Cancer. 2018. PMID: 29743050 Free PMC article. Review.
-
The Next Immune-Checkpoint Inhibitors: PD-1/PD-L1 Blockade in Melanoma.Clin Ther. 2015 Apr 1;37(4):764-82. doi: 10.1016/j.clinthera.2015.02.018. Epub 2015 Mar 29. Clin Ther. 2015. PMID: 25823918 Free PMC article. Review.
Cited by
-
The Diagnosis and Management of Cutaneous Metastases from Melanoma.Int J Mol Sci. 2023 Sep 26;24(19):14535. doi: 10.3390/ijms241914535. Int J Mol Sci. 2023. PMID: 37833981 Free PMC article. Review.
-
Metastatic patterns and treatment options for head and neck cutaneous squamous cell carcinoma (Review).Mol Clin Oncol. 2024 Apr 24;20(6):40. doi: 10.3892/mco.2024.2739. eCollection 2024 Jun. Mol Clin Oncol. 2024. PMID: 38756868 Free PMC article. Review.
-
Calcium electroporation induces tumor eradication, long-lasting immunity and cytokine responses in the CT26 colon cancer mouse model.Oncoimmunology. 2017 Mar 17;6(5):e1301332. doi: 10.1080/2162402X.2017.1301332. eCollection 2017. Oncoimmunology. 2017. PMID: 28638724 Free PMC article.
-
Review of Role of Surgery with Electroporation in Melanoma: Chemotherapy, Immunotherapy, and Gene Delivery.J Clin Med. 2024 Jun 29;13(13):3828. doi: 10.3390/jcm13133828. J Clin Med. 2024. PMID: 38999394 Free PMC article. Review.
-
Electroporation in Head-and-Neck Cancer: An Innovative Approach with Immunotherapy and Nanotechnology Combination.Cancers (Basel). 2022 Oct 31;14(21):5363. doi: 10.3390/cancers14215363. Cancers (Basel). 2022. PMID: 36358782 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

