Molecular characterization of circulating tumor cells in ovarian cancer
- PMID: 27293992
- PMCID: PMC4889713
Molecular characterization of circulating tumor cells in ovarian cancer
Abstract
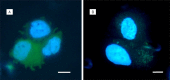
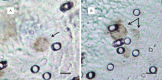
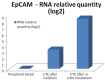
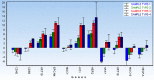
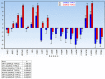
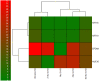
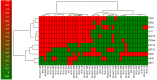
The main focus of the study was to detect circulating tumor cells (CTCs) in ovarian cancer (OC) patients using a new methodological approach (MetaCell(TM)) which is based on size-dependent separation of CTCs and subsequent cytomorphological evaluation. Cytomorphological evaluation using vital fluorescence microscopy approach enables to use the captured cells for further RNA/DNA analysis. The cytomorphological analysis is then completed by gene expression analysis (GEA). GEA showed that relative expression of EPCAM is elevated in CTC-enriched fractions in comparison to the whole peripheral blood sample and that the expression grows with in vitro cultivation time. Comparison of the relative gene expression level in the group of peripheral blood samples and CTC-fraction samples confirmed a statistically significant difference for the following genes (p < 0.02): KRT7, WT1, EPCAM, MUC16, MUC1, KRT18 and KRT19. Thus, we suggest that the combination of the above listed genes could confirm CTCs presence in OC patients with higher specificity than when GEA tests are performed for one marker only. The GEA revealed two separate clusters identifying patients with or without CTCs.
Keywords: CTCs; MetaCell; circulating tumor cells; cultivation; gene expression; in vitro; ovarian cancer.
Figures
Similar articles
-
Isolation, primary culture, morphological and molecular characterization of circulating tumor cells in gynecological cancers.Am J Transl Res. 2015 Jul 15;7(7):1203-13. eCollection 2015. Am J Transl Res. 2015. PMID: 26328005 Free PMC article.
-
Detection and cultivation of circulating tumor cells in gastric cancer.Cytotechnology. 2016 Aug;68(4):1095-102. doi: 10.1007/s10616-015-9866-9. Epub 2015 Apr 11. Cytotechnology. 2016. PMID: 25862542 Free PMC article.
-
The added value of circulating tumor cells examination in ovarian cancer staging.Am J Cancer Res. 2015 Oct 15;5(11):3363-75. eCollection 2015. Am J Cancer Res. 2015. PMID: 26807317 Free PMC article.
-
Current and future role of circulating tumor cells in patients with epithelial ovarian cancer.Eur J Surg Oncol. 2016 Dec;42(12):1772-1779. doi: 10.1016/j.ejso.2016.05.010. Epub 2016 May 25. Eur J Surg Oncol. 2016. PMID: 27265041 Review.
-
Enrichment, Isolation and Molecular Characterization of EpCAM-Negative Circulating Tumor Cells.Adv Exp Med Biol. 2017;994:181-203. doi: 10.1007/978-3-319-55947-6_10. Adv Exp Med Biol. 2017. PMID: 28560675 Review.
Cited by
-
Circulating cell-free DNA and circulating tumor cells, the "liquid biopsies" in ovarian cancer.J Ovarian Res. 2017 Nov 13;10(1):75. doi: 10.1186/s13048-017-0369-5. J Ovarian Res. 2017. PMID: 29132396 Free PMC article. Review.
-
Technologies for Viable Circulating Tumor Cell Isolation.Int J Mol Sci. 2022 Dec 15;23(24):15979. doi: 10.3390/ijms232415979. Int J Mol Sci. 2022. PMID: 36555625 Free PMC article. Review.
-
Quantification of Wilms' tumor 1 mRNA by digital polymerase chain reaction.Int J Hematol. 2018 Feb;107(2):230-234. doi: 10.1007/s12185-017-2336-8. Epub 2017 Oct 9. Int J Hematol. 2018. PMID: 28994041
-
Circulating tumor cells: what we know, what do we want to know about them and are they ready to be used in clinics?Am J Transl Res. 2017 Jun 15;9(6):2807-2823. eCollection 2017. Am J Transl Res. 2017. PMID: 28670371 Free PMC article.
-
Extracellular vesicle contents as non-invasive biomarkers in ovarian malignancies.Mol Ther Oncolytics. 2022 Aug 5;26:347-359. doi: 10.1016/j.omto.2022.08.005. eCollection 2022 Sep 15. Mol Ther Oncolytics. 2022. PMID: 36090475 Free PMC article. Review.
References
-
- Josse SA, Pantel K. Biologic challenges in the detection of circulating tumor cells. Cancer Res. 2013;73:8–11. - PubMed
-
- Ning Y, Gerger A, Zhang W, Hanna DL, Yang D, Winder T, Wakatsuki T, Labonte MJ, Stintzing S, Volz N, Sunakawa Y, Stremitzer S, El-Khoueiry R, Lenz HJ. Plastin polymorphisms predict genderand stage-specific colon cancer recurrence after adjuvant chemotherapy. Mol Cancer Ther. 2014;13:528–39. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
