Threshold-Dependent Cooperativity of Pdx1 and Oc1 in Pancreatic Progenitors Establishes Competency for Endocrine Differentiation and β-Cell Function
- PMID: 27292642
- PMCID: PMC4917419
- DOI: 10.1016/j.celrep.2016.05.040
Threshold-Dependent Cooperativity of Pdx1 and Oc1 in Pancreatic Progenitors Establishes Competency for Endocrine Differentiation and β-Cell Function
Abstract
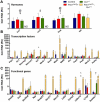
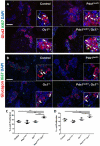
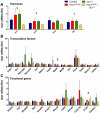
Pdx1 and Oc1 are co-expressed in multipotent pancreatic progenitors and regulate the pro-endocrine gene Neurog3. Their expression diverges in later organogenesis, with Oc1 absent from hormone+ cells and Pdx1 maintained in mature β cells. In a classical genetic test for cooperative functional interactions, we derived mice with combined Pdx1 and Oc1 heterozygosity. Endocrine development in double-heterozygous pancreata was normal at embryonic day (E)13.5, but defects in specification and differentiation were apparent at E15.5, the height of the second wave of differentiation. Pancreata from double heterozygotes showed alterations in the expression of genes crucial for β-cell development and function, decreased numbers and altered allocation of Neurog3-expressing endocrine progenitors, and defective endocrine differentiation. Defects in islet gene expression and β-cell function persisted in double heterozygous neonates. These results suggest that Oc1 and Pdx1 cooperate prior to their divergence, in pancreatic progenitors, to allow for proper differentiation and functional maturation of β cells.
Published by Elsevier Inc.
Figures
Similar articles
-
Cooperative function of Pdx1 and Oc1 in multipotent pancreatic progenitors impacts postnatal islet maturation and adaptability.Am J Physiol Endocrinol Metab. 2018 Apr 1;314(4):E308-E321. doi: 10.1152/ajpendo.00260.2017. Epub 2017 Dec 12. Am J Physiol Endocrinol Metab. 2018. PMID: 29351489 Free PMC article.
-
The transcription factors Nkx6.1 and Nkx6.2 possess equivalent activities in promoting beta-cell fate specification in Pdx1+ pancreatic progenitor cells.Development. 2007 Jul;134(13):2491-500. doi: 10.1242/dev.002691. Epub 2007 May 30. Development. 2007. PMID: 17537793
-
The mammal-specific Pdx1 Area II enhancer has multiple essential functions in early endocrine cell specification and postnatal β-cell maturation.Development. 2017 Jan 15;144(2):248-257. doi: 10.1242/dev.143123. Epub 2016 Dec 19. Development. 2017. PMID: 27993987 Free PMC article.
-
PDX1, Neurogenin-3, and MAFA: critical transcription regulators for beta cell development and regeneration.Stem Cell Res Ther. 2017 Nov 2;8(1):240. doi: 10.1186/s13287-017-0694-z. Stem Cell Res Ther. 2017. PMID: 29096722 Free PMC article. Review.
-
Direct lineage tracing reveals the ontogeny of pancreatic cell fates during mouse embryogenesis.Mech Dev. 2003 Jan;120(1):35-43. doi: 10.1016/s0925-4773(02)00330-1. Mech Dev. 2003. PMID: 12490294 Review.
Cited by
-
Connective tissue growth factor is critical for proper β-cell function and pregnancy-induced β-cell hyperplasia in adult mice.Am J Physiol Endocrinol Metab. 2016 Sep 1;311(3):E564-74. doi: 10.1152/ajpendo.00194.2016. Epub 2016 Jul 26. Am J Physiol Endocrinol Metab. 2016. Retraction in: Am J Physiol Endocrinol Metab. 2017 Jul 1;313(1):E105. doi: 10.1152/ajpendo.zh1-7764-retr.2017. PMID: 27460898 Free PMC article. Retracted.
-
Transcriptional changes and the role of ONECUT1 in hPSC pancreatic differentiation.Commun Biol. 2021 Nov 17;4(1):1298. doi: 10.1038/s42003-021-02818-3. Commun Biol. 2021. PMID: 34789845 Free PMC article.
-
[Research progress on the donor cell sources of pancreatic islet transplantation for treatment of diabetes mellitus].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2018 Jan 15;32(1):104-111. doi: 10.7507/1002-1892.201707049. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2018. PMID: 29806374 Free PMC article. Review. Chinese.
-
Cooperative function of Pdx1 and Oc1 in multipotent pancreatic progenitors impacts postnatal islet maturation and adaptability.Am J Physiol Endocrinol Metab. 2018 Apr 1;314(4):E308-E321. doi: 10.1152/ajpendo.00260.2017. Epub 2017 Dec 12. Am J Physiol Endocrinol Metab. 2018. PMID: 29351489 Free PMC article.
-
Pancreatic Cancer: Molecular Characterization, Clonal Evolution and Cancer Stem Cells.Biomedicines. 2017 Nov 18;5(4):65. doi: 10.3390/biomedicines5040065. Biomedicines. 2017. PMID: 29156578 Free PMC article. Review.
References
-
- Brissova M, Shiota M, Nicholson WE, Gannon M, Knobel SM, Piston DW, Wright CV, Powers AC. Reduction in pancreatic transcription factor PDX-1 impairs glucose-stimulated insulin secretion. J. Biol. Chem. 2002;277:11225–11232. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K12 DK094723/DK/NIDDK NIH HHS/United States
- R01 DK105689/DK/NIDDK NIH HHS/United States
- G20 RR030956/RR/NCRR NIH HHS/United States
- K12 HD000850/HD/NICHD NIH HHS/United States
- P30 EY008126/EY/NEI NIH HHS/United States
- R01 DK122039/DK/NIDDK NIH HHS/United States
- T32 DK007563/DK/NIDDK NIH HHS/United States
- P30 DK019525/DK/NIDDK NIH HHS/United States
- T32 HD007502/HD/NICHD NIH HHS/United States
- P60 DK020593/DK/NIDDK NIH HHS/United States
- R01 DK068157/DK/NIDDK NIH HHS/United States
- U01 DK089570/DK/NIDDK NIH HHS/United States
- U01 DK089540/DK/NIDDK NIH HHS/United States
- I01 BX000666/BX/BLRD VA/United States
- P30 DK020593/DK/NIDDK NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- I01 BX000990/BX/BLRD VA/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

