Connexin 50 Regulates Surface Ball-and-Socket Structures and Fiber Cell Organization
- PMID: 27281269
- PMCID: PMC4913802
- DOI: 10.1167/iovs.16-19521
Connexin 50 Regulates Surface Ball-and-Socket Structures and Fiber Cell Organization
Abstract
Purpose: The roles of gap junction protein connexin 50 (Cx50) encoded by Gja8, during lens development are not fully understood. Connexin 50 knockout (KO) lenses have decreased proliferation of epithelial cells and altered fiber cell denucleation. We further investigated the mechanism for cellular defects in Cx50 KO (Gja8-/-) lenses.
Methods: Fiber cell morphology and subcellular distribution of various lens membrane/cytoskeleton proteins from wild-type and Cx50 KO mice were visualized by immunofluorescent staining and confocal microscopy.
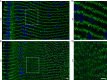
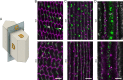
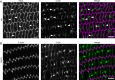
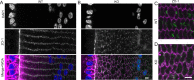
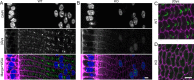
Results: We observed multiple morphological defects in the cortical fibers of Cx50 KO lenses, including abnormal fiber cell packing geometry, decreased F-actin enrichment at tricellular vertices, and disrupted ball-and-socket (BS) structures on the long sides of hexagonal fibers. Moreover, only small gap junction plaques consisting of Cx46 (α3 connexin) were detected in cortical fibers and the distributions of the BS-associated beta-dystroglycan and ZO-1 proteins were altered.
Conclusions: Connexin 50 gap junctions are important for BS structure maturation and cortical fiber cell organization. Connexin 50-based gap junction plaques likely form structural domains with an array of membrane/cytoskeletal proteins to stabilize BS. Loss of Cx50-mediated coupling, BS disruption, and altered F-actin in Cx50 KO fibers, thereby contribute to the small lens and mild cataract phenotypes.
Figures
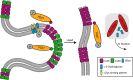
Similar articles
-
Knock-in of Cx46 partially rescues fiber defects in lenses lacking Cx50.Mol Vis. 2017 Mar 24;23:160-170. eCollection 2017. Mol Vis. 2017. PMID: 28458505 Free PMC article.
-
Lens ion homeostasis relies on the assembly and/or stability of large connexin 46 gap junction plaques on the broad sides of differentiating fiber cells.Am J Physiol Cell Physiol. 2015 May 15;308(10):C835-47. doi: 10.1152/ajpcell.00372.2014. Epub 2015 Mar 4. Am J Physiol Cell Physiol. 2015. PMID: 25740157 Free PMC article.
-
Lens gap junctional coupling is modulated by connexin identity and the locus of gene expression.Invest Ophthalmol Vis Sci. 2004 Oct;45(10):3629-37. doi: 10.1167/iovs.04-0445. Invest Ophthalmol Vis Sci. 2004. PMID: 15452070
-
Gap junctions or hemichannel-dependent and independent roles of connexins in cataractogenesis and lens development.Curr Mol Med. 2010 Dec;10(9):851-63. doi: 10.2174/156652410793937750. Curr Mol Med. 2010. PMID: 21091421 Free PMC article. Review.
-
Focus on lens connexins.BMC Cell Biol. 2017 Jan 17;18(Suppl 1):6. doi: 10.1186/s12860-016-0116-6. BMC Cell Biol. 2017. PMID: 28124626 Free PMC article. Review.
Cited by
-
Generation of Lens Progenitor Cells and Lentoid Bodies from Pluripotent Stem Cells: Novel Tools for Human Lens Development and Ocular Disease Etiology.Cells. 2022 Nov 6;11(21):3516. doi: 10.3390/cells11213516. Cells. 2022. PMID: 36359912 Free PMC article. Review.
-
Age-related changes of lens stiffness in wild-type and Cx46 knockout mice.Exp Eye Res. 2021 Nov;212:108777. doi: 10.1016/j.exer.2021.108777. Epub 2021 Sep 29. Exp Eye Res. 2021. PMID: 34597677 Free PMC article.
-
Connexin 50 and AQP0 are Essential in Maintaining Organization and Integrity of Lens Fibers.Invest Ophthalmol Vis Sci. 2019 Sep 3;60(12):4021-4032. doi: 10.1167/iovs.18-26270. Invest Ophthalmol Vis Sci. 2019. PMID: 31560767 Free PMC article.
-
Charged multivesicular body protein 4b forms complexes with gap junction proteins during lens fiber cell differentiation.FASEB J. 2023 Apr;37(4):e22801. doi: 10.1096/fj.202201368RR. FASEB J. 2023. PMID: 36880430 Free PMC article.
-
Biochemical and biomechanical characteristics of dystrophin-deficient mdx3cv mouse lens.Biochim Biophys Acta Mol Basis Dis. 2021 Jan 1;1867(1):165998. doi: 10.1016/j.bbadis.2020.165998. Epub 2020 Oct 27. Biochim Biophys Acta Mol Basis Dis. 2021. PMID: 33127476 Free PMC article.
References
-
- Kuszak JR,, Zoltoski RK,, Sivertson C. Fibre cell organization in crystalline lenses. Exp Eye Res. 2004; 78: 673–687. - PubMed
-
- Mochizuki T,, Masai I. The lens equator: a platform for molecular machinery that regulates the switch from cell proliferation to differentiation in the vertebrate lens. Dev Growth Differ. 2014; 56: 387–401. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

