Persistence With Conventional Triple Therapy Versus a Tumor Necrosis Factor Inhibitor and Methotrexate in US Veterans With Rheumatoid Arthritis
- PMID: 27273801
- PMCID: PMC6207907
- DOI: 10.1002/acr.22944
Persistence With Conventional Triple Therapy Versus a Tumor Necrosis Factor Inhibitor and Methotrexate in US Veterans With Rheumatoid Arthritis
Abstract
Objective: To compare persistence and adherence to triple therapy with the nonbiologic disease-modifying antirheumatic drugs (DMARDs) methotrexate (MTX), hydroxychloroquine, and sulfasalazine, versus a tumor necrosis factor inhibitor (TNFi) plus MTX in patients with rheumatoid arthritis (RA).
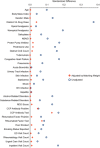
Methods: Administrative and laboratory data were analyzed for US Veterans with RA initiating triple therapy or TNFi + MTX between January 2006 and December 2012. Treatment persistence 365 days postindex was calculated using 3 definitions. Definition 1 required no gap in therapy of ≥90 days for any drug in the original combination. Definition 2 required no added or switched DMARD, no decrease to nonbiologic DMARD monotherapy, and no termination of all DMARD therapies. Definition 3 was similar to definition 2 but allowed a switch to another drug within the same class. Adherence used a proportion of days covered of ≥80%. Propensity-weighted analysis with matched weights was used to balance covariates.
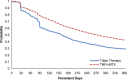
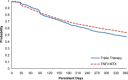
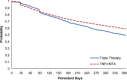
Results: The analysis included 4,364 RA patients (TNFi + MTX, n = 3,204; triple therapy, n = 1,160). In propensity-weighted analysis, patients in the TNFi + MTX group were significantly more likely than patients in the triple therapy group to satisfy all persistence criteria in definition 1 (risk difference [RD] 13.1% [95% confidence interval (95% CI) 9.2-17.0]), definition 2 (RD 6.4% [95% CI 2.3-10.5]), and definition 3 (RD 9.5% [95% CI 5.5-13.6]). Patients in the TNFi + MTX group also exhibited higher adherence during the first year (RD 7.2% [95% CI 3.8-10.5]).
Conclusion: US Veterans with RA were significantly more likely to be persistent and adherent to combination therapy with TNFi + MTX than triple therapy with nonbiologic DMARDs.
© 2016, American College of Rheumatology.
Figures
Similar articles
-
Real-World Outcomes Associated With Methotrexate, Sulfasalazine, and Hydroxychloroquine Triple Therapy Versus Tumor Necrosis Factor Inhibitor/Methotrexate Combination Therapy in Patients With Rheumatoid Arthritis.Arthritis Care Res (Hoboken). 2021 Aug;73(8):1114-1124. doi: 10.1002/acr.24253. Arthritis Care Res (Hoboken). 2021. PMID: 32374918
-
Etanercept-Methotrexate Combination Therapy Initiators Have Greater Adherence and Persistence Than Triple Therapy Initiators With Rheumatoid Arthritis.Arthritis Care Res (Hoboken). 2015 Dec;67(12):1656-63. doi: 10.1002/acr.22638. Arthritis Care Res (Hoboken). 2015. PMID: 26097194 Free PMC article.
-
Examining Time to Initiation of Biologic Disease-modifying Antirheumatic Drugs and Medication Adherence and Persistence Among Texas Medicaid Recipients With Rheumatoid Arthritis.Clin Ther. 2016 Mar;38(3):646-54. doi: 10.1016/j.clinthera.2016.01.022. Epub 2016 Feb 18. Clin Ther. 2016. PMID: 26899313
-
Drug combinations with methotrexate to treat rheumatoid arthritis.Clin Exp Rheumatol. 2010 Sep-Oct;28(5 Suppl 61):S52-7. Epub 2010 Oct 28. Clin Exp Rheumatol. 2010. PMID: 21044434 Review.
-
The role of concomitant methotrexate in biologic therapy for rheumatoid arthritis.Bull Hosp Jt Dis (2013). 2013;71 Suppl 1:S29-32. Bull Hosp Jt Dis (2013). 2013. PMID: 24219038 Review.
Cited by
-
Hydroxychloroquine retinopathy.Eye (Lond). 2017 Jun;31(6):828-845. doi: 10.1038/eye.2016.298. Epub 2017 Mar 10. Eye (Lond). 2017. PMID: 28282061 Free PMC article. Review.
-
2021 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis.Arthritis Care Res (Hoboken). 2021 Jul;73(7):924-939. doi: 10.1002/acr.24596. Epub 2021 Jun 8. Arthritis Care Res (Hoboken). 2021. PMID: 34101387 Free PMC article.
-
Turkish League Against Rheumatism (TLAR) Recommendations for the Pharmacological Management of Rheumatoid Arthritis: 2018 Update Under Guidance of Current Recommendations.Arch Rheumatol. 2018 Jul 9;33(3):251-271. doi: 10.5606/ArchRheumatol.2018.6911. eCollection 2018 Sep. Arch Rheumatol. 2018. PMID: 30632540 Free PMC article.
-
Negative central venous to arterial lactate gradient in patients receiving vasopressors is associated with higher ICU 30-day mortality: a retrospective cohort study.BMC Anesthesiol. 2021 Jan 22;21(1):25. doi: 10.1186/s12871-021-01237-5. BMC Anesthesiol. 2021. PMID: 33482733 Free PMC article.
-
Adherence of rheumatoid arthritis patients to biologic disease-modifying antirheumatic drugs: a cross-sectional study.Rheumatol Int. 2017 Oct;37(10):1709-1718. doi: 10.1007/s00296-017-3758-6. Epub 2017 Jun 19. Rheumatol Int. 2017. PMID: 28631046
References
-
- Helmick CG, Felson DT, Lawrence RC, Gabriel S, Hirsch R, Kwoh CK, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: part I. Arthritis Rheum 2008;58:15–25. - PubMed
-
- Sacks JJ, Luo YH, Helmick CG. Prevalence of specific types of arthritis and other rheumatic conditions in the ambulatory health care system in the United States, 2001–2005. Arthritis Care Res (Hoboken) 2010;62:460–4. - PubMed
-
- Saag KG, Teng GG, Patkar NM, Anuntiyo J, Finney C, Curtis JR, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease‐modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum 2008;59:762–84. - PubMed
-
- Singh JA, Furst DE, Bharat A, Curtis JR, Kavanaugh AF, Kremer JM, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease‐modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64:625–39. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

