VEGF-ablation therapy reduces drug delivery and therapeutic response in ECM-dense tumors
- PMID: 27270432
- PMCID: PMC5237662
- DOI: 10.1038/onc.2016.182
VEGF-ablation therapy reduces drug delivery and therapeutic response in ECM-dense tumors
Abstract
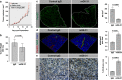
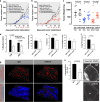
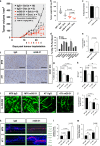
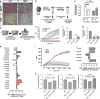
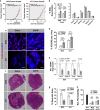
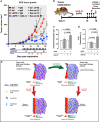
The inadequate transport of drugs into the tumor tissue caused by its abnormal vasculature is a major obstacle to the treatment of cancer. Anti-vascular endothelial growth factor (anti-VEGF) drugs can cause phenotypic alteration and maturation of the tumor's vasculature. However, whether this consistently improves delivery and subsequent response to therapy is still controversial. Clinical results indicate that not all patients benefit from antiangiogenic treatment, necessitating the development of criteria to predict the effect of these agents in individual tumors. We demonstrate that, in anti-VEGF-refractory murine tumors, vascular changes after VEGF ablation result in reduced delivery leading to therapeutic failure. In these tumors, the impaired response after anti-VEGF treatment is directly linked to strong deposition of fibrillar extracellular matrix (ECM) components and high expression of lysyl oxidases. The resulting condensed, highly crosslinked ECM impeded drug permeation, protecting tumor cells from exposure to small-molecule drugs. The reduced vascular density after anti-VEGF treatment further decreased delivery in these tumors, an effect not compensated by the improved vessel quality. Pharmacological inhibition of lysyl oxidases improved drug delivery in various tumor models and reversed the negative effect of VEGF ablation on drug delivery and therapeutic response in anti-VEGF-resistant tumors. In conclusion, the vascular changes after anti-VEGF therapy can have a context-dependent negative impact on overall therapeutic efficacy. A determining factor is the tumor ECM, which strongly influences the effect of anti-VEGF therapy. Our results reveal the prospect to revert a possible negative effect and to potentiate responsiveness to antiangiogenic therapy by concomitantly targeting ECM-modifying enzymes.
Figures
Similar articles
-
Mediators of glioblastoma resistance and invasion during antivascular endothelial growth factor therapy.Clin Cancer Res. 2009 Jul 15;15(14):4589-99. doi: 10.1158/1078-0432.CCR-09-0575. Epub 2009 Jun 30. Clin Cancer Res. 2009. PMID: 19567589
-
Generation of Potent Anti-Vascular Endothelial Growth Factor Neutralizing Antibodies from Mouse Phage Display Library for Cancer Therapy.Int J Mol Sci. 2016 Feb 5;17(2):214. doi: 10.3390/ijms17020214. Int J Mol Sci. 2016. PMID: 26861297 Free PMC article.
-
Tumor angiogenesis and accessibility: role of vascular endothelial growth factor.Semin Oncol. 2002 Dec;29(6 Suppl 16):3-9. doi: 10.1053/sonc.2002.37265. Semin Oncol. 2002. PMID: 12516032 Review.
-
Inhibition of Lysyl Oxidases Improves Drug Diffusion and Increases Efficacy of Cytotoxic Treatment in 3D Tumor Models.Sci Rep. 2015 Dec 1;5:17576. doi: 10.1038/srep17576. Sci Rep. 2015. PMID: 26620400 Free PMC article.
-
Monoclonal antibodies targeting vascular endothelial growth factor: current status and future challenges in cancer therapy.BioDrugs. 2009;23(5):289-304. doi: 10.2165/11317600-000000000-00000. BioDrugs. 2009. PMID: 19754219 Review.
Cited by
-
Progression after discontinuation of bevacizumab in the first-line treatment of ovarian cancer.Ann Transl Med. 2023 Mar 15;11(5):229. doi: 10.21037/atm-22-6389. Epub 2023 Jan 29. Ann Transl Med. 2023. PMID: 37007537 Free PMC article. No abstract available.
-
TME-Related Biomimetic Strategies Against Cancer.Int J Nanomedicine. 2024 Jan 4;19:109-135. doi: 10.2147/IJN.S441135. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38192633 Free PMC article. Review.
-
Extracellular Matrix in the Tumor Microenvironment and Its Impact on Cancer Therapy.Front Mol Biosci. 2020 Jan 31;6:160. doi: 10.3389/fmolb.2019.00160. eCollection 2019. Front Mol Biosci. 2020. PMID: 32118030 Free PMC article. Review.
-
Nanoparticle-mediated TRPV1 channel blockade amplifies cancer thermo-immunotherapy via heat shock factor 1 modulation.Nat Commun. 2023 Apr 29;14(1):2498. doi: 10.1038/s41467-023-38128-x. Nat Commun. 2023. PMID: 37120615 Free PMC article.
-
Harnessing Extracellular Matrix Biology for Tumor Drug Delivery.J Pers Med. 2021 Jan 31;11(2):88. doi: 10.3390/jpm11020088. J Pers Med. 2021. PMID: 33572559 Free PMC article. Review.
References
-
- Kratz F. Drug delivery in oncology − challenges and perspectives. In: Kratz F, Steinhagen H, Senter P (eds). Drug Delivery in Oncology − Challenges and Perspectives in Drug Delivery in Oncology – from Research Concepts to Cancer Therapy, vol. 1. VCM: Weinheim, Germany; 2011, pp LIX-LXXXV.
-
- Gangloff A, Hsueh WA, Kesner AL, Kiesewetter DO, Pio BS, Pegram MD et al. Estimation of paclitaxel biodistribution and uptake in human-derived xenografts in vivo with (18)F-fluoropaclitaxel. J Nucl Med 2005; 46: 1866–1871. - PubMed
-
- Kesner AL, Hsueh WA, Htet NL, Pio BS, Czernin J, Pegram MD et al. Biodistribution and predictive value of 18 F-fluorocyclophosphamide in mice bearing human breast cancer xenografts. J Nucl Med 2007; 48: 2021–2027. - PubMed
-
- Staffhorst RW, van der Born K, Erkelens CA, Hamelers IH, Peters GJ, Boven E et al. Antitumor activity and biodistribution of cisplatin nanocapsules in nude mice bearing human ovarian carcinoma xenografts. Anticancer Drugs 2008; 19: 721–727. - PubMed
-
- Memon AA, Jakobsen S, Dagnaes-Hansen F, Sorensen BS, Keiding S, Nexo E. Positron emission tomography (PET) imaging with [11C]-labeled erlotinib: a micro-PET study on mice with lung tumor xenografts. Cancer Res 2009; 69: 873–878. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

