Hypo- and Hyper-Assembly Diseases of RNA-Protein Complexes
- PMID: 27263464
- PMCID: PMC4925306
- DOI: 10.1016/j.molmed.2016.05.005
Hypo- and Hyper-Assembly Diseases of RNA-Protein Complexes
Abstract
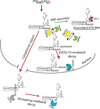
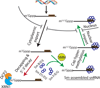
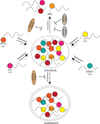
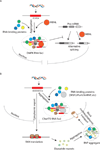
A key aspect of cellular function is the proper assembly and utilization of ribonucleoproteins (RNPs). Recent studies have shown that hyper- or hypo-assembly of various RNPs can lead to human diseases. Defects in the formation of RNPs lead to 'RNP hypo-assembly diseases', which can be caused by RNA degradation outcompeting RNP assembly. By contrast, excess RNP assembly, either in higher order RNP granules, or due to the expression of repeat-containing RNAs, can lead to 'RNP hyper-assembly diseases'. Here, we discuss the most recent advances in understanding the cause of disease onset, as well as potential therapies from the aspect of modulating RNP assembly in the cell, which presents a novel route to the treatment of these diseases.
Keywords: RNA quality control; RNP assembly; dyskeratosis congenita; spinal muscular atrophy; stress granules; therapy.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures
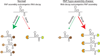
Similar articles
-
Unraveling the pathogenesis of Hoyeraal-Hreidarsson syndrome, a complex telomere biology disorder.Br J Haematol. 2015 Aug;170(4):457-71. doi: 10.1111/bjh.13442. Epub 2015 May 4. Br J Haematol. 2015. PMID: 25940403 Free PMC article. Review.
-
Pathogenic NAP57 mutations decrease ribonucleoprotein assembly in dyskeratosis congenita.Hum Mol Genet. 2009 Dec 1;18(23):4546-51. doi: 10.1093/hmg/ddp416. Epub 2009 Sep 4. Hum Mol Genet. 2009. PMID: 19734544 Free PMC article.
-
Dyskeratosis congenita mutations in the H/ACA domain of human telomerase RNA affect its assembly into a pre-RNP.RNA. 2009 Feb;15(2):235-43. doi: 10.1261/rna.1354009. Epub 2008 Dec 17. RNA. 2009. PMID: 19095616 Free PMC article.
-
Cartilage hair hypoplasia and celiac disease: report of an Indian girl with novel genotype.Indian J Gastroenterol. 2013 Nov;32(6):409-12. doi: 10.1007/s12664-013-0358-6. Epub 2013 Aug 17. Indian J Gastroenterol. 2013. PMID: 23949991
-
RNP Assembly Defects in Spinal Muscular Atrophy.Adv Neurobiol. 2018;20:143-171. doi: 10.1007/978-3-319-89689-2_6. Adv Neurobiol. 2018. PMID: 29916019 Review.
Cited by
-
A crystal structure of a collaborative RNA regulatory complex reveals mechanisms to refine target specificity.Elife. 2019 Aug 9;8:e48968. doi: 10.7554/eLife.48968. Elife. 2019. PMID: 31397673 Free PMC article.
-
RNA biology of disease-associated microsatellite repeat expansions.Acta Neuropathol Commun. 2017 Aug 29;5(1):63. doi: 10.1186/s40478-017-0468-y. Acta Neuropathol Commun. 2017. PMID: 28851463 Free PMC article. Review.
-
Axonal mRNA localization and local translation in neurodegenerative disease.Neural Regen Res. 2021 Oct;16(10):1950-1957. doi: 10.4103/1673-5374.308074. Neural Regen Res. 2021. PMID: 33642365 Free PMC article. Review.
-
Chromatin-contact atlas reveals disorder-mediated protein interactions and moonlighting chromatin-associated RBPs.Nucleic Acids Res. 2021 Dec 16;49(22):13092-13107. doi: 10.1093/nar/gkab1180. Nucleic Acids Res. 2021. PMID: 34871434 Free PMC article.
-
Reactivation of nonsense-mediated mRNA decay protects against C9orf72 dipeptide-repeat neurotoxicity.Brain. 2019 May 1;142(5):1349-1364. doi: 10.1093/brain/awz070. Brain. 2019. PMID: 30938419 Free PMC article.
References
-
- Müller-McNicoll M, Neugebauer KM. How cells get the message: dynamic assembly and function of mRNA–protein complexes. Nature Reviews Genetics. 2013;14:275–287. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

