Molecular mechanism and functional significance of acid generation in the Drosophila midgut
- PMID: 27250760
- PMCID: PMC4890030
- DOI: 10.1038/srep27242
Molecular mechanism and functional significance of acid generation in the Drosophila midgut
Abstract
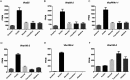
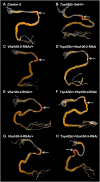
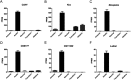
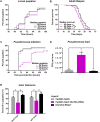
The gut of Drosophila melanogaster includes a proximal acidic region (~pH 2), however the genome lacks the H(+)/K(+) ATPase characteristic of the mammalian gastric parietal cell, and the molecular mechanisms of acid generation are poorly understood. Here, we show that maintenance of the low pH of the acidic region is dependent on H(+) V-ATPase, together with carbonic anhydrase and five further transporters or channels that mediate K(+), Cl(-) and HCO3(-) transport. Abrogation of the low pH did not influence larval survival under standard laboratory conditions, but was deleterious for insects subjected to high Na(+) or K(+) load. Insects with elevated pH in the acidic region displayed increased susceptibility to Pseudomonas pathogens and increased abundance of key members of the gut microbiota (Acetobacter and Lactobacillus), suggesting that the acidic region has bacteriostatic or bacteriocidal activity. Conversely, the pH of the acidic region was significantly reduced in germ-free Drosophila, indicative of a role of the gut bacteria in shaping the pH conditions of the gut. These results demonstrate that the acidic gut region protects the insect and gut microbiome from pathological disruption, and shed light on the mechanisms by which low pH can be maintained in the absence of H(+), K(+) ATPase.
Figures



Similar articles
-
The roles of V-type H+-ATPase and Na+/K+-ATPase in energizing K+ and H+ transport in larval Drosophila gut epithelia.J Insect Physiol. 2017 Apr;98:284-290. doi: 10.1016/j.jinsphys.2017.01.019. Epub 2017 Feb 7. J Insect Physiol. 2017. PMID: 28188726
-
Reduced Gut Acidity Induces an Obese-Like Phenotype in Drosophila melanogaster and in Mice.PLoS One. 2015 Oct 5;10(10):e0139722. doi: 10.1371/journal.pone.0139722. eCollection 2015. PLoS One. 2015. PMID: 26436771 Free PMC article.
-
Cationic pathway of pH regulation in larvae of Anopheles gambiae.J Exp Biol. 2008 Mar;211(Pt 6):957-68. doi: 10.1242/jeb.012021. J Exp Biol. 2008. PMID: 18310121
-
Epithelial ultrastructure and cellular mechanisms of acid and base transport in the Drosophila midgut.J Exp Biol. 2009 Jun;212(Pt 11):1731-44. doi: 10.1242/jeb.029306. J Exp Biol. 2009. PMID: 19448082 Review.
-
Genetic diseases of acid-base transporters.Annu Rev Physiol. 2002;64:899-923. doi: 10.1146/annurev.physiol.64.092801.141759. Annu Rev Physiol. 2002. PMID: 11826292 Review.
Cited by
-
Factors Related to Bacillus thuringiensis and Gut Physiology. Comment on Rajan, V. An Alkaline Foregut Protects Herbivores from Latex in Forage, but Increases Their Susceptibility to Bt Endotoxin. Life 2023, 13, 2195.Life (Basel). 2024 Jan 31;14(2):205. doi: 10.3390/life14020205. Life (Basel). 2024. PMID: 38398714 Free PMC article.
-
Spatiotemporally Heterogeneous Population Dynamics of Gut Bacteria Inferred from Fecal Time Series Data.mBio. 2018 Jan 9;9(1):e01453-17. doi: 10.1128/mBio.01453-17. mBio. 2018. PMID: 29317508 Free PMC article.
-
An intestinal zinc sensor regulates food intake and developmental growth.Nature. 2020 Apr;580(7802):263-268. doi: 10.1038/s41586-020-2111-5. Epub 2020 Mar 18. Nature. 2020. PMID: 32269334 Free PMC article.
-
The evolution of sour taste.Proc Biol Sci. 2022 Feb 9;289(1968):20211918. doi: 10.1098/rspb.2021.1918. Epub 2022 Feb 9. Proc Biol Sci. 2022. PMID: 35135352 Free PMC article. Review.
-
Epithelial Cell Polarity During Drosophila Midgut Development.Front Cell Dev Biol. 2022 Jun 30;10:886773. doi: 10.3389/fcell.2022.886773. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35846367 Free PMC article. Review.
References
-
- Yao X. & Forte J. G. Cell biology of acid secretion by the parietal cell. Annu Rev Physiol 65, 103–131 (2003). - PubMed
-
- Grahammer F. et al. The cardiac K+ channel KCNQ1 is essential for gastric acid secretion. Gastroenterology 120, 1363–1371 (2001). - PubMed
-
- Malinowska D. H., Kupert E. Y., Bahinski A., Sherry A. M. & Cuppoletti J. Cloning, functional expression, and characterization of a PKA-activated gastric Cl-channel. Am J Physiol 268, C191–200 (1995). - PubMed
-
- Stuart-Tilley A., Sardet C., Pouyssegur J., Schwartz M. A., Brown D. & Alper S. L. Immunolocalization of anion exchanger AE2 and cation exchanger NHE-1 in distinct adjacent cells of gastric mucosa. Am J Physiol 266, C559–568 (1994). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

