IL-10-dependent Tr1 cells attenuate astrocyte activation and ameliorate chronic central nervous system inflammation
- PMID: 27246324
- PMCID: PMC4939696
- DOI: 10.1093/brain/aww113
IL-10-dependent Tr1 cells attenuate astrocyte activation and ameliorate chronic central nervous system inflammation
Abstract
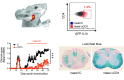
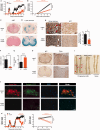
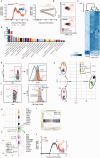
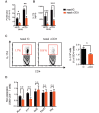
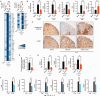
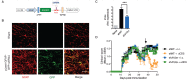
SEE WINGER AND ZAMVIL DOI101093/BRAIN/AWW121 FOR A SCIENTIFIC COMMENTARY ON THIS ARTICLE: The innate immune system plays a central role in the chronic central nervous system inflammation that drives neurological disability in progressive forms of multiple sclerosis, for which there are no effective treatments. The mucosal immune system is a unique tolerogenic organ that provides a physiological approach for the induction of regulatory T cells. Here we report that nasal administration of CD3-specific antibody ameliorates disease in a progressive animal model of multiple sclerosis. This effect is IL-10-dependent and is mediated by the induction of regulatory T cells that share a similar transcriptional profile to Tr1 regulatory cells and that suppress the astrocyte inflammatory transcriptional program. Treatment results in an attenuated inflammatory milieu in the central nervous system, decreased microglia activation, reduced recruitment of peripheral monocytes, stabilization of the blood-brain barrier and less neurodegeneration. These findings suggest a new therapeutic approach for the treatment of progressive forms of multiple sclerosis and potentially other types of chronic central nervous system inflammation.
Keywords: T-lymphocytes; astrocyte; interleukin 10; multiple sclerosis; neuroinflammation.
© The Author (2016). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures



Comment in
-
Your nose knows how to target brain inflammation.Brain. 2016 Jul;139(Pt 7):1866-9. doi: 10.1093/brain/aww121. Brain. 2016. PMID: 27343218 Free PMC article.
Similar articles
-
γδ T Cell-Secreted XCL1 Mediates Anti-CD3-Induced Oral Tolerance.J Immunol. 2019 Nov 15;203(10):2621-2629. doi: 10.4049/jimmunol.1900784. Epub 2019 Oct 2. J Immunol. 2019. PMID: 31578268 Free PMC article.
-
1,25-dihydroxyvitamin D3 -induced dendritic cells suppress experimental autoimmune encephalomyelitis by increasing proportions of the regulatory lymphocytes and reducing T helper type 1 and type 17 cells.Immunology. 2017 Nov;152(3):414-424. doi: 10.1111/imm.12776. Epub 2017 Jul 10. Immunology. 2017. PMID: 28617989 Free PMC article.
-
Astrocyte response to IFN-γ limits IL-6-mediated microglia activation and progressive autoimmune encephalomyelitis.J Neuroinflammation. 2015 Apr 22;12:79. doi: 10.1186/s12974-015-0293-9. J Neuroinflammation. 2015. PMID: 25896970 Free PMC article.
-
Cisplatin induces tolerogenic dendritic cells in response to TLR agonists via the abundant production of IL-10, thereby promoting Th2- and Tr1-biased T-cell immunity.Oncotarget. 2016 Jun 7;7(23):33765-82. doi: 10.18632/oncotarget.9260. Oncotarget. 2016. PMID: 27172902 Free PMC article.
-
Astrocytes in multiple sclerosis and experimental autoimmune encephalomyelitis: Star-shaped cells illuminating the darkness of CNS autoimmunity.Brain Behav Immun. 2019 Aug;80:10-24. doi: 10.1016/j.bbi.2019.05.029. Epub 2019 May 21. Brain Behav Immun. 2019. PMID: 31125711 Review.
Cited by
-
Genetic insights into the relationship between immune cell characteristics and ischemic stroke: A bidirectional Mendelian randomization study.Eur J Neurol. 2024 May;31(5):e16226. doi: 10.1111/ene.16226. Epub 2024 Feb 7. Eur J Neurol. 2024. PMID: 38323746 Free PMC article.
-
Good Things Come in Threes: Genetically Engineered Neural Stem Cells Mitigate Chronic CNS Autoimmunity.Mol Ther. 2016 Aug;24(8):1338-9. doi: 10.1038/mt.2016.157. Mol Ther. 2016. PMID: 27578282 Free PMC article. No abstract available.
-
Autoimmune encephalomyelitis in NOD mice is not initially a progressive multiple sclerosis model.Ann Clin Transl Neurol. 2019 Aug;6(8):1362-1372. doi: 10.1002/acn3.792. Epub 2019 Jul 15. Ann Clin Transl Neurol. 2019. PMID: 31402611 Free PMC article.
-
Nanomodulation of Macrophages in Multiple Sclerosis.Cells. 2019 Jun 5;8(6):543. doi: 10.3390/cells8060543. Cells. 2019. PMID: 31195710 Free PMC article. Review.
-
Role of orally induced regulatory T cells in immunotherapy and tolerance.Cell Immunol. 2021 Jan;359:104251. doi: 10.1016/j.cellimm.2020.104251. Epub 2020 Nov 14. Cell Immunol. 2021. PMID: 33248367 Free PMC article. Review.
References
-
- Allan SE, Broady R, Gregori S, Himmel ME, Locke N, Roncarolo MG, et al. . CD4+ T-regulatory cells: toward therapy for human diseases . Immunol Rev 2008. ; 223 : 391 – 421 . - PubMed
-
- Alvarez JI, Dodelet-Devillers A, Kebir H, Ifergan I, Fabre PJ, Terouz S, et al. . The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence . Science 2011. ; 334 : 1727 – 31 . - PubMed
-
- Anderson AC, Anderson DE, Bregoli L, Hastings WD, Kassam N, Lei C, et al. . Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells . Science 2007. ; 318 : 1141 – 3 . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

