Implementation of coordinated global serotype 2 oral poliovirus vaccine cessation: risks of inadvertent trivalent oral poliovirus vaccine use
- PMID: 27246198
- PMCID: PMC4888482
- DOI: 10.1186/s12879-016-1537-8
Implementation of coordinated global serotype 2 oral poliovirus vaccine cessation: risks of inadvertent trivalent oral poliovirus vaccine use
Abstract
Background: The endgame for polio eradication includes coordinated global cessation of oral poliovirus vaccine (OPV), starting with the cessation of vaccine containing OPV serotype 2 (OPV2) by switching all trivalent OPV (tOPV) to bivalent OPV (bOPV). The logistics associated with this global switch represent a significant undertaking, with some possibility of inadvertent tOPV use after the switch.
Methods: We used a previously developed poliovirus transmission and OPV evolution model to explore the relationships between the extent of inadvertent tOPV use, the time after the switch of the inadvertent tOPV use and corresponding population immunity to serotype 2 poliovirus transmission, and the ability of the inadvertently introduced viruses to cause a serotype 2 circulating vaccine-derived poliovirus (cVDPV2) outbreak in a hypothetical population. We then estimated the minimum time until inadvertent tOPV use in a supplemental immunization activity (SIA) or in routine immunization (RI) can lead to a cVDPV2 outbreak in realistic populations with properties like those of northern India, northern Pakistan and Afghanistan, northern Nigeria, and Ukraine.
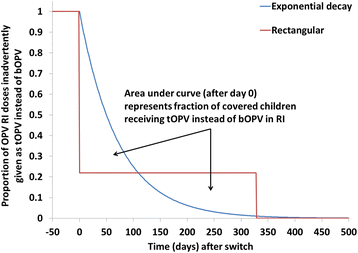
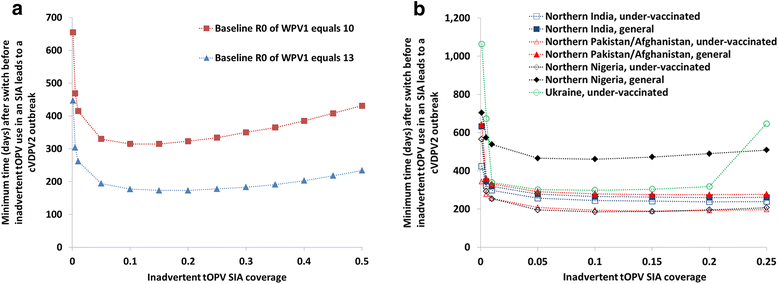
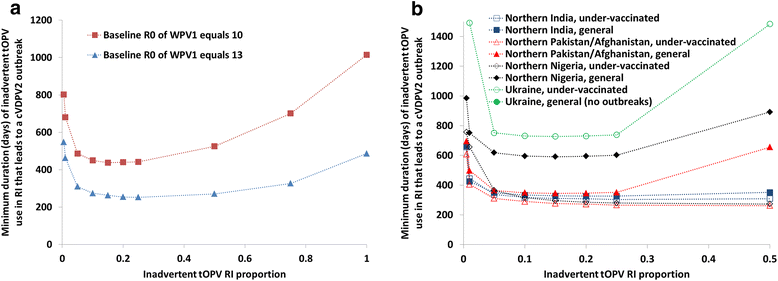
Results: At low levels of inadvertent tOPV use, the minimum time after the switch for the inadvertent use to cause a cVDPV2 outbreak decreases sharply with increasing proportions of children inadvertently receiving tOPV. The minimum times until inadvertent tOPV use in an SIA or in RI can lead to a cVDPV2 outbreak varies widely among populations, with higher basic reproduction numbers, lower tOPV-induced population immunity to serotype 2 poliovirus transmission prior to the switch, and a lower proportion of transmission occurring via the oropharyngeal route all resulting in shorter times. In populations with the lowest expected immunity to serotype 2 poliovirus transmission after the switch, inadvertent tOPV use in an SIA leads to a cVDPV2 outbreak if it occurs as soon as 9 months after the switch with 0.5 % of children aged 0-4 years inadvertently receiving tOPV, and as short as 6 months after the switch with 10-20 % of children aged 0-1 years inadvertently receiving tOPV. In the same populations, inadvertent tOPV use in RI leads to a cVDPV2 outbreak if 0.5 % of OPV RI doses given use tOPV instead of bOPV for at least 20 months after the switch, with the minimum length of use dropping to at least 9 months if inadvertent tOPV use occurs in 50 % of OPV RI doses.
Conclusions: Efforts to ensure timely and complete tOPV withdrawal at all levels, particularly from locations storing large amounts of tOPV, will help minimize risks associated with the tOPV-bOPV switch. Under-vaccinated populations with poor hygiene become at risk of a cVDPV2 outbreak in the event of inadvertent tOPV use the soonest after the tOPV-bOPV switch and therefore should represent priority areas to ensure tOPV withdrawal from all OPV stocks.
Keywords: Dynamic modeling; Eradication; Oral poliovirus vaccine; Polio; Risk management; Vaccine-derived poliovirus.
Figures
Similar articles
-
Implementation of coordinated global serotype 2 oral poliovirus vaccine cessation: risks of potential non-synchronous cessation.BMC Infect Dis. 2016 May 26;16:231. doi: 10.1186/s12879-016-1536-9. BMC Infect Dis. 2016. PMID: 27230071 Free PMC article.
-
The differential impact of oral poliovirus vaccine formulation choices on serotype-specific population immunity to poliovirus transmission.BMC Infect Dis. 2015 Sep 17;15:376. doi: 10.1186/s12879-015-1116-4. BMC Infect Dis. 2015. PMID: 26382234 Free PMC article.
-
Introduction of Inactivated Poliovirus Vaccine and Switch from Trivalent to Bivalent Oral Poliovirus Vaccine - Worldwide, 2013-2016.MMWR Morb Mortal Wkly Rep. 2015 Jul 3;64(25):699-702. MMWR Morb Mortal Wkly Rep. 2015. PMID: 26135591 Free PMC article.
-
Polio endgame: the global switch from tOPV to bOPV.Expert Rev Vaccines. 2016 Jun;15(6):693-708. doi: 10.1586/14760584.2016.1140041. Epub 2016 Feb 2. Expert Rev Vaccines. 2016. PMID: 26751187 Review.
-
Updated Characterization of Post-OPV Cessation Risks: Lessons from 2019 Serotype 2 Outbreaks and Implications for the Probability of OPV Restart.Risk Anal. 2021 Feb;41(2):320-328. doi: 10.1111/risa.13555. Epub 2020 Jul 6. Risk Anal. 2021. PMID: 32632925 Free PMC article. Review.
Cited by
-
Increasing Population Immunity Prior to Globally-Coordinated Cessation of Bivalent Oral Poliovirus Vaccine (bOPV).Pathogens. 2024 Sep 17;13(9):804. doi: 10.3390/pathogens13090804. Pathogens. 2024. PMID: 39338995 Free PMC article.
-
From vaccine to pathogen: Modeling Sabin 2 vaccine virus reversion and evolutionary epidemiology in Matlab, Bangladesh.Virus Evol. 2023 Jul 8;9(2):vead044. doi: 10.1093/ve/vead044. eCollection 2023. Virus Evol. 2023. PMID: 37692896 Free PMC article.
-
Complexity of options related to restarting oral poliovirus vaccine (OPV) in national immunization programs after OPV cessation.Gates Open Res. 2023 Apr 17;7:55. doi: 10.12688/gatesopenres.14511.1. eCollection 2023. Gates Open Res. 2023. PMID: 37547300 Free PMC article.
-
Coordinated global cessation of oral poliovirus vaccine use: Options and potential consequences.Risk Anal. 2024 Feb;44(2):366-378. doi: 10.1111/risa.14158. Epub 2023 Jun 21. Risk Anal. 2024. PMID: 37344934 Free PMC article. Review.
-
Worst-case scenarios: Modeling uncontrolled type 2 polio transmission.Risk Anal. 2024 Feb;44(2):379-389. doi: 10.1111/risa.14159. Epub 2023 Jun 21. Risk Anal. 2024. PMID: 37344376 Free PMC article.
References
-
- World Health Assembly . Poliomyelitis (resolution 68.3) Geneva: World Health Organization; 2015.
-
- World Health Organization: Global Polio Eradication Initiative: Polio Eradication and Endgame Strategic Plan (2013–2018). Report number: WHO/POLIO/13.02. Geneva; 2013.
-
- Global Polio Eradication Initiative; Global eradication of wild poliovirus type 2 declared [http://www.polioeradication.org/mediaroom/newsstories/Global-eradication..., accessed 30 Nov 2015]
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

