The Differential DRP1 Phosphorylation and Mitochondrial Dynamics in the Regional Specific Astroglial Death Induced by Status Epilepticus
- PMID: 27242436
- PMCID: PMC4870264
- DOI: 10.3389/fncel.2016.00124
The Differential DRP1 Phosphorylation and Mitochondrial Dynamics in the Regional Specific Astroglial Death Induced by Status Epilepticus
Abstract
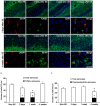
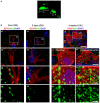
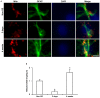
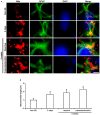
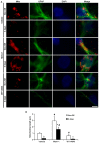
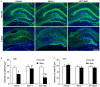
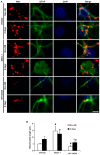
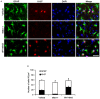
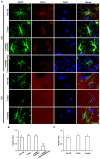
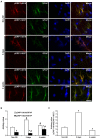
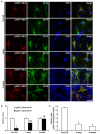
The response and susceptibility to astroglial degenerations are relevant to the distinctive properties of astrocytes in a hemodynamic-independent manner following status epilepticus (SE). Since impaired mitochondrial fission plays an important role in mitosis, apoptosis and programmed necrosis, we investigated whether the unique pattern of mitochondrial dynamics is involved in the characteristics of astroglial death induced by SE. In the present study, SE induced astroglial apoptosis in the molecular layer of the dentate gyrus, accompanied by decreased mitochondrial length. In contrast, clasmatodendritic (autophagic) astrocytes in the CA1 region showed mitochondrial elongation induced by SE. Mdivi-1 (an inhibitor of mitochondrial fission) effectively attenuated astroglial apoptosis, but WY14643 (an enhancer of mitochondrial fission) aggravated it. In addition, Mdivi-1 accelerated clasmatodendritic changes in astrocytes. These regional specific mitochondrial dynamics in astrocytes were closely correlated with dynamin-related protein 1 (DRP1; a mitochondrial fission protein) phosphorylation, not optic atrophy 1 (OPA1; a mitochondrial fusion protein) expression. To the best of our knowledge, the present data demonstrate for the first time the novel role of DRP1-mediated mitochondrial fission in astroglial loss. Thus, the present findings suggest that the differential astroglial mitochondrial dynamics may participate in the distinct characteristics of astroglial death induced by SE.
Keywords: DRP1; astroglial death; clasmatodendrosis; mitochondria; status epilepticus.
Figures
Similar articles
-
CDK5 inhibitors prevent astroglial apoptosis and reactive astrogliosis by regulating PKA and DRP1 phosphorylations in the rat hippocampus.Neurosci Res. 2017 Jun;119:24-37. doi: 10.1016/j.neures.2017.01.006. Epub 2017 Jan 30. Neurosci Res. 2017. PMID: 28153522
-
CDDO-Me Attenuates Clasmatodendrosis in CA1 Astrocyte by Inhibiting HSP25-AKT Mediated DRP1-S637 Phosphorylation in Chronic Epilepsy Rats.Int J Mol Sci. 2022 Apr 20;23(9):4569. doi: 10.3390/ijms23094569. Int J Mol Sci. 2022. PMID: 35562960 Free PMC article.
-
CDDO-Me Abrogates Aberrant Mitochondrial Elongation in Clasmatodendritic Degeneration by Regulating NF-κB-PDI-Mediated S-Nitrosylation of DRP1.Int J Mol Sci. 2023 Mar 20;24(6):5875. doi: 10.3390/ijms24065875. Int J Mol Sci. 2023. PMID: 36982949 Free PMC article.
-
The role of Drp1 adaptor proteins MiD49 and MiD51 in mitochondrial fission: implications for human disease.Clin Sci (Lond). 2016 Nov 1;130(21):1861-74. doi: 10.1042/CS20160030. Clin Sci (Lond). 2016. PMID: 27660309 Review.
-
To mdivi-1 or not to mdivi-1: Is that the question?Dev Neurobiol. 2017 Nov;77(11):1260-1268. doi: 10.1002/dneu.22519. Epub 2017 Aug 30. Dev Neurobiol. 2017. PMID: 28842943 Free PMC article. Review.
Cited by
-
Amyloid-β accumulation in human astrocytes induces mitochondrial disruption and changed energy metabolism.J Neuroinflammation. 2023 Feb 20;20(1):43. doi: 10.1186/s12974-023-02722-z. J Neuroinflammation. 2023. PMID: 36803838 Free PMC article.
-
UGCG overexpression leads to increased glycolysis and increased oxidative phosphorylation of breast cancer cells.Sci Rep. 2020 May 18;10(1):8182. doi: 10.1038/s41598-020-65182-y. Sci Rep. 2020. PMID: 32424263 Free PMC article.
-
Mitochondrial Dynamics, Mitophagy, and Mitochondria-Endoplasmic Reticulum Contact Sites Crosstalk Under Hypoxia.Front Cell Dev Biol. 2022 Feb 25;10:848214. doi: 10.3389/fcell.2022.848214. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35281107 Free PMC article. Review.
-
CDDO-Me Attenuates Astroglial Autophagy via Nrf2-, ERK1/2-SP1- and Src-CK2-PTEN-PI3K/AKT-Mediated Signaling Pathways in the Hippocampus of Chronic Epilepsy Rats.Antioxidants (Basel). 2021 Apr 23;10(5):655. doi: 10.3390/antiox10050655. Antioxidants (Basel). 2021. PMID: 33922531 Free PMC article.
-
Astroglial role in the pathophysiology of status epilepticus: an overview.Oncotarget. 2018 Jun 1;9(42):26954-26976. doi: 10.18632/oncotarget.25485. eCollection 2018 Jun 1. Oncotarget. 2018. PMID: 29928494 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

