Current status and perspectives in translational biomarker research for PD-1/PD-L1 immune checkpoint blockade therapy
- PMID: 27234522
- PMCID: PMC4884396
- DOI: 10.1186/s13045-016-0277-y
Current status and perspectives in translational biomarker research for PD-1/PD-L1 immune checkpoint blockade therapy
Abstract
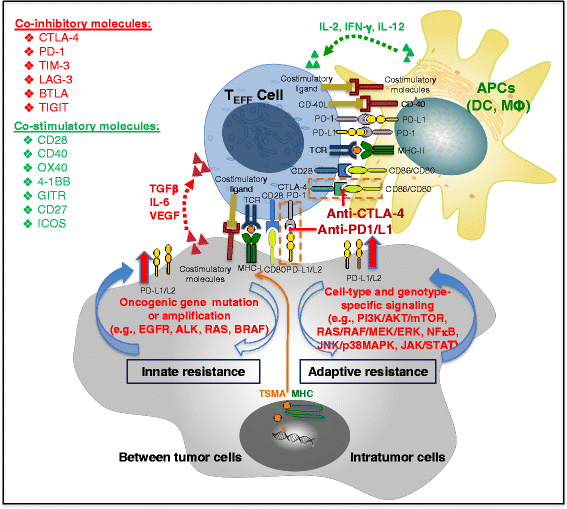
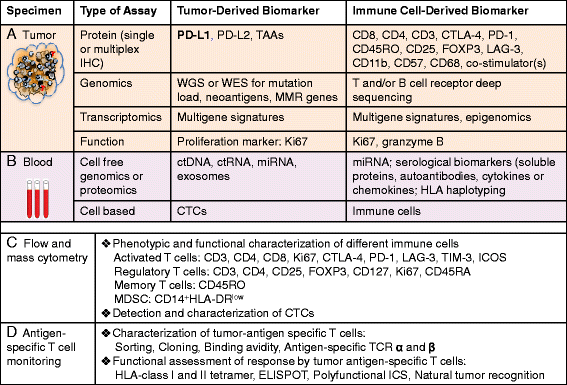
Modulating immune inhibitory pathways has been a major recent breakthrough in cancer treatment. Checkpoint blockade antibodies targeting cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programed cell-death protein 1 (PD-1) have demonstrated acceptable toxicity, promising clinical responses, durable disease control, and improved survival in some patients with advanced melanoma, non-small cell lung cancer (NSCLC), and other tumor types. About 20 % of advanced NSCLC patients and 30 % of advanced melanoma patients experience tumor responses from checkpoint blockade monotherapy, with better clinical responses seen with the combination of anti-PD-1 and anti-CTLA-4 antibodies. Given the power of these new therapies, it is important to understand the complex and dynamic nature of host immune responses and the regulation of additional molecules in the tumor microenvironment and normal organs in response to the checkpoint blockade therapies. In this era of precision oncology, there remains a largely unmet need to identify the patients who are most likely to benefit from immunotherapy, to optimize the monitoring assays for tumor-specific immune responses, to develop strategies to improve clinical efficacy, and to identify biomarkers so that immune-related adverse events can be avoided. At this time, PD-L1 immunohistochemistry (IHC) staining using 22C3 antibody is the only FDA-approved companion diagnostic for patients with NSCLC-treated pembrolizumab, but more are expected to come to market. We here summarize the current knowledge, clinical efficacy, potential immune biomarkers, and associated assays for immune checkpoint blockade therapies in advanced solid tumors.
Keywords: Biomarker; Cancer immunotherapy; Cytotoxic T cells; Immune checkpoint blockade antibodies; Immune-related adverse events; PD-1; PD-L1; Precision oncology.
Figures
Similar articles
-
The Next Immune-Checkpoint Inhibitors: PD-1/PD-L1 Blockade in Melanoma.Clin Ther. 2015 Apr 1;37(4):764-82. doi: 10.1016/j.clinthera.2015.02.018. Epub 2015 Mar 29. Clin Ther. 2015. PMID: 25823918 Free PMC article. Review.
-
PD-1/PD-L1 Blockade Therapy in Advanced Non-Small-Cell Lung Cancer: Current Status and Future Directions.Oncologist. 2019 Feb;24(Suppl 1):S31-S41. doi: 10.1634/theoncologist.2019-IO-S1-s05. Oncologist. 2019. PMID: 30819829 Free PMC article. Review.
-
Immune Checkpoint Blockade in Breast Cancer Therapy.Adv Exp Med Biol. 2017;1026:383-402. doi: 10.1007/978-981-10-6020-5_18. Adv Exp Med Biol. 2017. PMID: 29282694 Review.
-
Association of Survival and Immune-Related Biomarkers With Immunotherapy in Patients With Non-Small Cell Lung Cancer: A Meta-analysis and Individual Patient-Level Analysis.JAMA Netw Open. 2019 Jul 3;2(7):e196879. doi: 10.1001/jamanetworkopen.2019.6879. JAMA Netw Open. 2019. PMID: 31290993 Free PMC article.
-
The next generation of immunotherapy: keeping lung cancer in check.J Hematol Oncol. 2017 Apr 24;10(1):87. doi: 10.1186/s13045-017-0456-5. J Hematol Oncol. 2017. PMID: 28434399 Free PMC article. Review.
Cited by
-
A CT-Based Radiomics Approach to Predict Nivolumab Response in Advanced Non-Small-Cell Lung Cancer.Front Oncol. 2021 Feb 24;11:544339. doi: 10.3389/fonc.2021.544339. eCollection 2021. Front Oncol. 2021. PMID: 33718125 Free PMC article.
-
Cancer neoantigens as potential targets for immunotherapy.Clin Exp Metastasis. 2022 Feb;39(1):51-60. doi: 10.1007/s10585-021-10091-1. Epub 2021 May 5. Clin Exp Metastasis. 2022. PMID: 33950415 Free PMC article. Review.
-
A Review of Emerging Biomarkers for Immune Checkpoint Inhibitors in Tumors of the Gastrointestinal Tract.Med Sci Monit. 2022 Feb 5;28:e935348. doi: 10.12659/MSM.935348. Med Sci Monit. 2022. PMID: 35121724 Free PMC article. Review.
-
Deep learning for predicting immunotherapeutic efficacy in advanced non-small cell lung cancer patients: a retrospective study combining progression-free survival risk and overall survival risk.Transl Lung Cancer Res. 2022 Apr;11(4):670-685. doi: 10.21037/tlcr-22-244. Transl Lung Cancer Res. 2022. PMID: 35529789 Free PMC article.
-
Silencing of A-kinase anchor protein 4 inhibits the metastasis and growth of non-small cell lung cancer.Bioengineered. 2022 Mar;13(3):6895-6907. doi: 10.1080/21655979.2021.1977105. Bioengineered. 2022. PMID: 35253625 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

