The involvement of TRPV1 in emesis and anti-emesis
- PMID: 27227028
- PMCID: PMC4843889
- DOI: 10.1080/23328940.2015.1043042
The involvement of TRPV1 in emesis and anti-emesis
Abstract
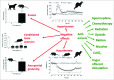
Diverse transmitter systems (e.g. acetylcholine, dopamine, endocannabinoids, endorphins, glutamate, histamine, 5-hydroxytryptamine, substance P) have been implicated in the pathways by which nausea and vomiting are induced and are targets for anti-emetic drugs (e.g. 5-hydroxytryptamine3 and tachykinin NK1 antagonists). The involvement of TRPV1 in emesis was discovered in the early 1990s and may have been overlooked previously as TRPV1 pharmacology was studied in rodents (mice, rats) lacking an emetic reflex. Acute subcutaneous administration of resiniferatoxin in the ferret, dog and Suncus murinus revealed that it had "broad-spectrum" anti-emetic effects against stimuli acting via both central (vestibular system, area postrema) and peripheral (abdominal vagal afferents) inputs. One of several hypotheses discussed here is that the anti-emetic effect is due to acute depletion of substance P (or another peptide) at a critical site (e.g. nucleus tractus solitarius) in the central emetic pathway. Studies in Suncus murinus revealed a potential for a long lasting (one month) effect against the chemotherapeutic agent cisplatin. Subsequent studies using telemetry in the conscious ferret compared the anti-emetic, hypothermic and hypertensive effects of resiniferatoxin (pungent) and olvanil (non-pungent) and showed that the anti-emetic effect was present (but reduced) with olvanil which although inducing hypothermia it did not have the marked hypertensive effects of resiniferatoxin. The review concludes by discussing general insights into emetic pathways and their pharmacology revealed by these relatively overlooked studies with TRPV1 activators (pungent an non-pungent; high and low lipophilicity) and antagonists and the potential clinical utility of agents targeted at the TRPV1 system.
Keywords: 12-HPETE, 12-hydroperoxy-eicosatetraenoic acid; 5-HT, 5-hydroxytryptamine; 5-HT3, 5-hdroxytryptamine3; 8-OH-DPAT, (±)-8-Hydroxy-2-dipropylaminotetralin; AM404; AM404, N-arachidonoylaminophenol; AMT, anandamide membrane transporter; AP, area postrema; BBB, blood brain barrier; CB1, cannabinoid1; CGRP, calcitonin gene-related peptide; CINV, chemotherapy-induced nausea and vomiting; CP 99,994; CTA, conditioned taste aversion; CVO's, circumventricular organs; D2, dopamine2; DRG, dorsal root ganglia; FAAH, fatty acid amide hydrolase; H1, histamine1; LTB4, leukotriene B4; NADA, N-arachidonoyl-dopamine; NK1, neurokinin1; POAH, preoptic anterior hypothalamus; RTX; Suncus murinus; TRPV1; TRPV1, transient receptor potential vanilloid receptor1; anti-emetic; capsaicin; ferret; i.v., intravenous; nausea; olvanil; thermoregulation; vanilloid; vomiting.
Figures

Similar articles
-
Olvanil: a non-pungent TRPV1 activator has anti-emetic properties in the ferret.Neuropharmacology. 2010 Feb;58(2):383-91. doi: 10.1016/j.neuropharm.2009.10.002. Epub 2009 Oct 13. Neuropharmacology. 2010. PMID: 19825380
-
Arvanil, anandamide and N-arachidonoyl-dopamine (NADA) inhibit emesis through cannabinoid CB1 and vanilloid TRPV1 receptors in the ferret.Eur J Neurosci. 2007 May;25(9):2773-82. doi: 10.1111/j.1460-9568.2007.05521.x. Epub 2007 Apr 25. Eur J Neurosci. 2007. PMID: 17459108
-
The emetic and anti-emetic effects of the capsaicin analogue resiniferatoxin in Suncus murinus, the house musk shrew.Br J Pharmacol. 2000 Jul;130(6):1247-54. doi: 10.1038/sj.bjp.0703428. Br J Pharmacol. 2000. PMID: 10903962 Free PMC article.
-
A History of Drug Discovery for Treatment of Nausea and Vomiting and the Implications for Future Research.Front Pharmacol. 2018 Sep 4;9:913. doi: 10.3389/fphar.2018.00913. eCollection 2018. Front Pharmacol. 2018. PMID: 30233361 Free PMC article. Review.
-
The abdominal visceral innervation and the emetic reflex: pathways, pharmacology, and plasticity.Can J Physiol Pharmacol. 1990 Feb;68(2):325-45. doi: 10.1139/y90-047. Can J Physiol Pharmacol. 1990. PMID: 2178756 Review.
Cited by
-
Cannabinoid Hyperemesis Syndrome Survey and Genomic Investigation.Cannabis Cannabinoid Res. 2022 Jun;7(3):336-344. doi: 10.1089/can.2021.0046. Epub 2021 Jul 5. Cannabis Cannabinoid Res. 2022. PMID: 34227878 Free PMC article.
-
Cannabinoid Hyperemesis Syndrome: A Review of Potential Mechanisms.Cannabis Cannabinoid Res. 2020 Jun 5;5(2):132-144. doi: 10.1089/can.2019.0059. eCollection 2020 Jun 1. Cannabis Cannabinoid Res. 2020. PMID: 32656345 Free PMC article. Review.
-
TRP (transient receptor potential) ion channel family: structures, biological functions and therapeutic interventions for diseases.Signal Transduct Target Ther. 2023 Jul 5;8(1):261. doi: 10.1038/s41392-023-01464-x. Signal Transduct Target Ther. 2023. PMID: 37402746 Free PMC article. Review.
-
Migraine inhibitor olcegepant reduces weight loss and IL-6 release in SARS-CoV-2-infected older mice with neurological signs.J Virol. 2024 Jul 23;98(7):e0006624. doi: 10.1128/jvi.00066-24. Epub 2024 May 30. J Virol. 2024. PMID: 38814068 Free PMC article.
-
Assessment of PDE4 Inhibitor-Induced Hypothermia as a Correlate of Nausea in Mice.Biology (Basel). 2021 Dec 20;10(12):1355. doi: 10.3390/biology10121355. Biology (Basel). 2021. PMID: 34943270 Free PMC article.
References
-
- Horn CC, Kimball BA, Wang H, Kaus J, Dienel S, Nagy A, Gathright GR, Yates BJ, Andrews PLR. Why can't rodents vomit? A comparative behavioral, anatomical, and physiological study. PLoS One 2013; 8:e60537; PMID:23593236; http://dx.doi.org/10.1371/journal.pone.0060537 - DOI - PMC - PubMed
-
- Sanger GJ, Broad J, Andrews PLR. The relationship between gastric motility and nausea: gastric prokinetic agents as treatments. Eur J Pharmacol 2013; 715:10-4; PMID:23831391; http://dx.doi.org/10.1016/j.ejphar.2013.06.031 - DOI - PubMed
-
- Andrews PLR, Sanger GJ. Nausea and the quest for the perfect anti-emetic. Eur J Pharmacol 2014; 722:108-21; PMID:24157981; http://dx.doi.org/10.1016/j.ejphar.2013.09.072 - DOI - PubMed
-
- Stern RM, Koch KL, Andrews PLR. Nausea: mechanisms and management. New York, U.S.A.: Oxford University Press; 2011.
-
- Borison HL, Wang SC. Physiology and pharmacology of vomiting. Pharmacol Rev 1953; 5(2):193-230; PMID:13064033 - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
