Peri-alloHCT IL-33 administration expands recipient T-regulatory cells that protect mice against acute GVHD
- PMID: 27222477
- PMCID: PMC4957164
- DOI: 10.1182/blood-2015-12-684142
Peri-alloHCT IL-33 administration expands recipient T-regulatory cells that protect mice against acute GVHD
Abstract
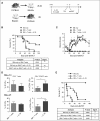
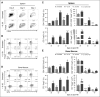
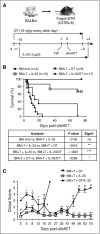
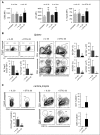
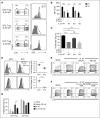
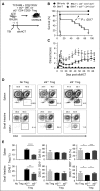
During allogeneic hematopoietic cell transplantation (alloHCT), nonhematopoietic cell interleukin-33 (IL-33) is augmented and released by recipient conditioning to promote type 1 alloimmunity and lethal acute graft-versus-host disease (GVHD). Yet, IL-33 is highly pleiotropic and exhibits potent immunoregulatory properties in the absence of coincident proinflammatory stimuli. We tested whether peri-alloHCT IL-33 delivery can protect against development of GVHD by augmenting IL-33-associated regulatory mechanisms. IL-33 administration augmented the frequency of regulatory T cells (Tregs) expressing the IL-33 receptor, suppression of tumorigenicity-2 (ST2), which persist following total body irradiation. ST2 expression is not exclusive to Tregs and IL-33 expands innate immune cells with regulatory or reparative properties. However, selective depletion of recipient Foxp3(+) cells concurrent with peri-alloHCT IL-33 administration accelerated acute GVHD lethality. IL-33-expanded Tregs protected recipients from GVHD by controlling macrophage activation and preventing accumulation of effector T cells in GVHD-target tissue. IL-33 stimulation of ST2 on Tregs activates p38 MAPK, which drives expansion of the ST2(+) Treg subset. Associated mechanistic studies revealed that proliferating Tregs exhibit IL-33-independent upregulation of ST2 and the adoptive transfer of st2(+) but not st2(-) Tregs mediated GVHD protection. In total, these data demonstrate the protective capacity of peri-alloHCT administration of IL-33 and IL-33-responsive Tregs in mouse models of acute GVHD. These findings provide strong support that the immunoregulatory relationship between IL-33 and Tregs can be harnessed therapeutically to prevent GVHD after alloHCT for treatment of malignancy or as a means for tolerance induction in solid organ transplantation.
© 2016 by The American Society of Hematology.
Figures

Similar articles
-
Rorc restrains the potency of ST2+ regulatory T cells in ameliorating intestinal graft-versus-host disease.JCI Insight. 2019 Mar 7;4(5):e122014. doi: 10.1172/jci.insight.122014. eCollection 2019 Mar 7. JCI Insight. 2019. PMID: 30694220 Free PMC article.
-
The IL-33/ST2 axis augments effector T-cell responses during acute GVHD.Blood. 2015 May 14;125(20):3183-92. doi: 10.1182/blood-2014-10-606830. Epub 2015 Mar 26. Blood. 2015. PMID: 25814531 Free PMC article.
-
IL-33 Receptor-Expressing Regulatory T Cells Are Highly Activated, Th2 Biased and Suppress CD4 T Cell Proliferation through IL-10 and TGFβ Release.PLoS One. 2016 Aug 22;11(8):e0161507. doi: 10.1371/journal.pone.0161507. eCollection 2016. PLoS One. 2016. PMID: 27548066 Free PMC article.
-
The role of regulatory T cells in graft-versus-host disease management.Expert Rev Hematol. 2020 Feb;13(2):141-154. doi: 10.1080/17474086.2020.1709436. Epub 2020 Jan 11. Expert Rev Hematol. 2020. PMID: 31874061 Review.
-
Controlling the burn and fueling the fire: defining the role for the alarmin interleukin-33 in alloimmunity.Curr Opin Organ Transplant. 2016 Feb;21(1):45-52. doi: 10.1097/MOT.0000000000000265. Curr Opin Organ Transplant. 2016. PMID: 26709577 Free PMC article. Review.
Cited by
-
A type 1 immunity-restricted promoter of the IL-33 receptor gene directs antiviral T-cell responses.Nat Immunol. 2024 Feb;25(2):256-267. doi: 10.1038/s41590-023-01697-6. Epub 2024 Jan 3. Nat Immunol. 2024. PMID: 38172258 Free PMC article.
-
ST2/MyD88 Deficiency Protects Mice against Acute Graft-versus-Host Disease and Spares Regulatory T Cells.J Immunol. 2019 May 15;202(10):3053-3064. doi: 10.4049/jimmunol.1800447. Epub 2019 Apr 12. J Immunol. 2019. PMID: 30979817 Free PMC article.
-
Emerging Roles of IL-33/ST2 Axis in Renal Diseases.Int J Mol Sci. 2017 Apr 7;18(4):783. doi: 10.3390/ijms18040783. Int J Mol Sci. 2017. PMID: 28387719 Free PMC article. Review.
-
Untangling Local Pro-Inflammatory, Reparative, and Regulatory Damage-Associated Molecular-Patterns (DAMPs) Pathways to Improve Transplant Outcomes.Front Immunol. 2021 Feb 23;12:611910. doi: 10.3389/fimmu.2021.611910. eCollection 2021. Front Immunol. 2021. PMID: 33708206 Free PMC article. Review.
-
Involvement of IL-33 in the Pathophysiology of Systemic Lupus Erythematosus: Review.Int J Mol Sci. 2022 Mar 15;23(6):3138. doi: 10.3390/ijms23063138. Int J Mol Sci. 2022. PMID: 35328556 Free PMC article. Review.
References
-
- Liew FY, Pitman NI, McInnes IB. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat Rev Immunol. 2010;10(2):103–110. - PubMed
-
- Cayrol C, Girard JP. IL-33: an alarmin cytokine with crucial roles in innate immunity, inflammation and allergy. Curr Opin Immunol. 2014;31:31–37. - PubMed
-
- Pichery M, Mirey E, Mercier P, et al. Endogenous IL-33 is highly expressed in mouse epithelial barrier tissues, lymphoid organs, brain, embryos, and inflamed tissues: in situ analysis using a novel Il-33-LacZ gene trap reporter strain. J Immunol. 2012;188(7):3488–3495. - PubMed
-
- Bonilla WV, Fröhlich A, Senn K, et al. The alarmin interleukin-33 drives protective antiviral CD8⁺ T cell responses. Science. 2012;335(6071):984–989. - PubMed
MeSH terms
Substances
Grants and funding
- T32 HL007062/HL/NHLBI NIH HHS/United States
- P01 CA065493/CA/NCI NIH HHS/United States
- R01 HL118979/HL/NHLBI NIH HHS/United States
- R01 HL122489/HL/NHLBI NIH HHS/United States
- T32 AI074490/AI/NIAID NIH HHS/United States
- R00 HL097155/HL/NHLBI NIH HHS/United States
- R01 AI034495/AI/NIAID NIH HHS/United States
- T32 GM008208/GM/NIGMS NIH HHS/United States
- R01 HL056067/HL/NHLBI NIH HHS/United States
- R01 CA072669/CA/NCI NIH HHS/United States
- P01 CA142106/CA/NCI NIH HHS/United States
- R21 AI121981/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

