Globally prevalent PfMDR1 mutations modulate Plasmodium falciparum susceptibility to artemisinin-based combination therapies
- PMID: 27189525
- PMCID: PMC4873939
- DOI: 10.1038/ncomms11553
Globally prevalent PfMDR1 mutations modulate Plasmodium falciparum susceptibility to artemisinin-based combination therapies
Abstract
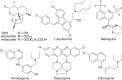
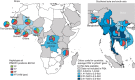
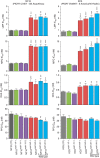
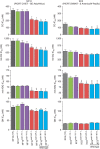
Antimalarial chemotherapy, globally reliant on artemisinin-based combination therapies (ACTs), is threatened by the spread of drug resistance in Plasmodium falciparum parasites. Here we use zinc-finger nucleases to genetically modify the multidrug resistance-1 transporter PfMDR1 at amino acids 86 and 184, and demonstrate that the widely prevalent N86Y mutation augments resistance to the ACT partner drug amodiaquine and the former first-line agent chloroquine. In contrast, N86Y increases parasite susceptibility to the partner drugs lumefantrine and mefloquine, and the active artemisinin metabolite dihydroartemisinin. The PfMDR1 N86 plus Y184F isoform moderately reduces piperaquine potency in strains expressing an Asian/African variant of the chloroquine resistance transporter PfCRT. Mutations in both digestive vacuole-resident transporters are thought to differentially regulate ACT drug interactions with host haem, a product of parasite-mediated haemoglobin degradation. Global mapping of these mutations illustrates where the different ACTs could be selectively deployed to optimize treatment based on regional differences in PfMDR1 haplotypes.
Figures

Similar articles
-
A Variant PfCRT Isoform Can Contribute to Plasmodium falciparum Resistance to the First-Line Partner Drug Piperaquine.mBio. 2017 May 9;8(3):e00303-17. doi: 10.1128/mBio.00303-17. mBio. 2017. PMID: 28487425 Free PMC article.
-
Expansion of a Specific Plasmodium falciparum PfMDR1 Haplotype in Southeast Asia with Increased Substrate Transport.mBio. 2020 Dec 1;11(6):e02093-20. doi: 10.1128/mBio.02093-20. mBio. 2020. PMID: 33262257 Free PMC article.
-
Selection of Plasmodium falciparum pfcrt and pfmdr1 polymorphisms after treatment with artesunate-amodiaquine fixed dose combination or artemether-lumefantrine in Liberia.Malar J. 2016 Sep 5;15(1):452. doi: 10.1186/s12936-016-1503-3. Malar J. 2016. PMID: 27596849 Free PMC article. Clinical Trial.
-
Molecular Mechanisms of Drug Resistance in Plasmodium falciparum Malaria.Annu Rev Microbiol. 2020 Sep 8;74:431-454. doi: 10.1146/annurev-micro-020518-115546. Annu Rev Microbiol. 2020. PMID: 32905757 Free PMC article. Review.
-
pfmdr1 (Plasmodium falciparum multidrug drug resistance gene 1): a pivotal factor in malaria resistance to artemisinin combination therapies.Expert Rev Anti Infect Ther. 2017 Jun;15(6):527-543. doi: 10.1080/14787210.2017.1313703. Epub 2017 Apr 10. Expert Rev Anti Infect Ther. 2017. PMID: 28355493 Review.
Cited by
-
A novel 4-aminoquinoline chemotype with multistage antimalarial activity and lack of cross-resistance with PfCRT and PfMDR1 mutants.PLoS Pathog. 2024 Oct 29;20(10):e1012627. doi: 10.1371/journal.ppat.1012627. eCollection 2024 Oct. PLoS Pathog. 2024. PMID: 39471233 Free PMC article.
-
Identification of the drug/metabolite transporter 1 as a marker of quinine resistance in a NF54×Cam3.II P. falciparum genetic cross.bioRxiv [Preprint]. 2024 Oct 1:2024.09.27.615529. doi: 10.1101/2024.09.27.615529. bioRxiv. 2024. PMID: 39386571 Free PMC article. Preprint.
-
Interchromosomal segmental duplication drives translocation and loss of P. falciparum histidine-rich protein 3.Elife. 2024 Oct 7;13:RP93534. doi: 10.7554/eLife.93534. Elife. 2024. PMID: 39373634 Free PMC article.
-
Molecular survey of pfmdr-1, pfcrt, and pfk13 gene mutations among patients returning from Plasmodium falciparum endemic areas to Turkey.Malar J. 2024 Sep 27;23(1):286. doi: 10.1186/s12936-024-05107-6. Malar J. 2024. PMID: 39334180 Free PMC article.
-
Investigation of Mutations in the crt-o and mdr1 Genes of Plasmodium vivax for the Molecular Surveillance of Chloroquine Resistance in Parasites from Gold Mining Areas in Roraima, Brazil.Microorganisms. 2024 Aug 15;12(8):1680. doi: 10.3390/microorganisms12081680. Microorganisms. 2024. PMID: 39203521 Free PMC article.
References
-
- WHO. Malaria World Report. http://www.who.int/malaria/publications/world-malaria-report-2015/en (2015).
-
- Klonis N., Creek D. J. & Tilley L. Iron and heme metabolism in Plasmodium falciparum and the mechanism of action of artemisinins. Curr. Opin. Microbiol. 16, 722–727 (2013). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

