Chronic expression of interferon-gamma leads to murine autoimmune cholangitis with a female predominance
- PMID: 27178326
- PMCID: PMC5033675
- DOI: 10.1002/hep.28641
Chronic expression of interferon-gamma leads to murine autoimmune cholangitis with a female predominance
Abstract
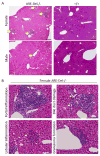
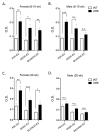
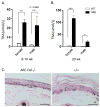
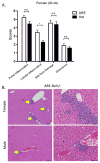
In most autoimmune diseases the serologic hallmarks of disease precede clinical pathology by years. Therefore, the use of animal models in defining early disease events becomes critical. We took advantage of a "designer" mouse with dysregulation of interferon gamma (IFNγ) characterized by prolonged and chronic expression of IFNγ through deletion of the IFNγ 3'-untranslated region adenylate uridylate-rich element (ARE). The ARE-Del(-/-) mice develop primary biliary cholangitis (PBC) with a female predominance that mimics human PBC that is characterized by up-regulation of total bile acids, spontaneous production of anti-mitochondrial antibodies, and portal duct inflammation. Transfer of CD4 T cells from ARE-Del(-/-) to B6/Rag1(-/-) mice induced moderate portal inflammation and parenchymal inflammation, and RNA sequencing of liver gene expression revealed that up-regulated genes potentially define early stages of cholangitis. Interestingly, up-regulated genes specifically overlap with the gene expression signature of biliary epithelial cells in PBC, implying that IFNγ may play a pathogenic role in biliary epithelial cells in the initiation stage of PBC. Moreover, differentially expressed genes in female mice have stronger type 1 and type 2 IFN signaling and lymphocyte-mediated immune responses and thus may drive the female bias of the disease.
Conclusion: Changes in IFNγ expression are critical for the pathogenesis of PBC. (Hepatology 2016;64:1189-1201).
© 2016 by the American Association for the Study of Liver Diseases. This article has been contributed to by U.S. Government employees and their work is in the public domain in the USA.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





Comment in
-
Mouse model of primary biliary cholangitis with a striking female predominance: A new powerful research tool.Hepatology. 2016 Oct;64(4):1024-7. doi: 10.1002/hep.28718. Epub 2016 Aug 2. Hepatology. 2016. PMID: 27388433 No abstract available.
Similar articles
-
The interplay of type I and type II interferons in murine autoimmune cholangitis as a basis for sex-biased autoimmunity.Hepatology. 2018 Apr;67(4):1408-1419. doi: 10.1002/hep.29524. Epub 2018 Feb 18. Hepatology. 2018. PMID: 28921595 Free PMC article.
-
Adoptive transfer of CD8(+) T cells from transforming growth factor beta receptor type II (dominant negative form) induces autoimmune cholangitis in mice.Hepatology. 2008 Jun;47(6):1974-82. doi: 10.1002/hep.22226. Hepatology. 2008. PMID: 18452147 Free PMC article.
-
Dual Roles of IFN-γ and IL-4 in the Natural History of Murine Autoimmune Cholangitis: IL-30 and Implications for Precision Medicine.Sci Rep. 2016 Oct 10;6:34884. doi: 10.1038/srep34884. Sci Rep. 2016. PMID: 27721424 Free PMC article.
-
The immunobiology of female predominance in primary biliary cholangitis.J Autoimmun. 2018 Dec;95:124-132. doi: 10.1016/j.jaut.2018.10.015. Epub 2018 Oct 25. J Autoimmun. 2018. PMID: 30509386 Review.
-
[Cholestatic liver diseases].Ther Umsch. 2004 Aug;61(8):521-7. doi: 10.1024/0040-5930.61.8.521. Ther Umsch. 2004. PMID: 15457969 Review. German.
Cited by
-
Endogenous IL-10 maintains immune tolerance but IL-10 gene transfer exacerbates autoimmune cholangitis.J Autoimmun. 2018 Dec;95:159-170. doi: 10.1016/j.jaut.2018.09.009. Epub 2018 Sep 28. J Autoimmun. 2018. PMID: 30274824 Free PMC article.
-
Integrated GWAS and mRNA Microarray Analysis Identified IFNG and CD40L as the Central Upstream Regulators in Primary Biliary Cholangitis.Hepatol Commun. 2020 Mar 15;4(5):724-738. doi: 10.1002/hep4.1497. eCollection 2020 May. Hepatol Commun. 2020. PMID: 32363322 Free PMC article.
-
Epigenetic disease markers in primary sclerosing cholangitis and primary biliary cholangitis-methylomics of cholestatic liver disease.Hepatol Commun. 2024 Jul 18;8(8):e0496. doi: 10.1097/HC9.0000000000000496. eCollection 2024 Aug 1. Hepatol Commun. 2024. PMID: 39023332 Free PMC article.
-
Autoimmunity affecting the biliary tract fuels the immunosurveillance of cholangiocarcinoma.J Exp Med. 2021 Oct 4;218(10):e20200853. doi: 10.1084/jem.20200853. Epub 2021 Sep 8. J Exp Med. 2021. PMID: 34495298 Free PMC article.
-
Deletion of Mcpip1 in Mcpip1fl/flAlbCre mice recapitulates the phenotype of human primary biliary cholangitis.Biochim Biophys Acta Mol Basis Dis. 2021 May 1;1867(5):166086. doi: 10.1016/j.bbadis.2021.166086. Epub 2021 Jan 26. Biochim Biophys Acta Mol Basis Dis. 2021. PMID: 33513427 Free PMC article.
References
-
- Floreani A, Franceschet I, Perini L, Cazzagon N, Gershwin ME, Bowlus CL. New therapies for primary biliary cirrhosis. Clinical reviews in allergy & immunology. 2015;48:263–272. - PubMed
-
- Gershwin ME, Mackay IR, Sturgess A, Coppel RL. Identification and specificity of a cDNA encoding the 70 kd mitochondrial antigen recognized in primary biliary cirrhosis. J Immunol. 1987;138:3525–3531. - PubMed
-
- Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med. 2005;353:1261–1273. - PubMed
-
- Beuers U, Gershwin ME, Gish RG, Invernizzi P, Jones DE, Lindor K, Ma X, et al. Changing nomenclature for PBC: From ‘cirrhosis’ to ‘cholangitis’. Digestive and liver disease: official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2015 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

