IL-4 production by group 2 innate lymphoid cells promotes food allergy by blocking regulatory T-cell function
- PMID: 27177780
- PMCID: PMC5014699
- DOI: 10.1016/j.jaci.2016.02.030
IL-4 production by group 2 innate lymphoid cells promotes food allergy by blocking regulatory T-cell function
Abstract
Background: Food allergy is a major health issue, but its pathogenesis remains obscure. Group 2 innate lymphoid cells (ILC2s) promote allergic inflammation. However their role in food allergy is largely unknown.
Objective: We sought to investigate the role of ILC2s in food allergy.
Methods: Food allergy-prone mice with a gain-of-function mutation in the IL-4 receptor α chain (Il4raF709) were orally sensitized with food allergens, and the ILC2 compartment was analyzed. The requirement for ILC2s in food allergy was investigated by using Il4raF709, IL-33 receptor-deficient (Il1rl1(-/-)), IL-13-deficient (Il13(-/-)), and IL-4-deficient (Il4(-/-)) mice and by adoptive transfer of in vitro-expanded ILC2s. Direct effects of ILC2s on regulatory T (Treg) cells and mast cells were analyzed in coculture experiments. Treg cell control of ILC2s was assessed in vitro and in vivo.
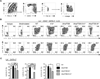
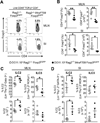
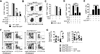
Results: Il4raF709 mice with food allergy exhibit increased numbers of ILC2s. IL-4 secretion by ILC2s contributes to the allergic response by reducing allergen-specific Treg cell and activating mast cell counts. IL-33 receptor deficiency in Il4raF709 Il1rl1(-/-) mice protects against allergen sensitization and anaphylaxis while reducing ILC2 induction. Adoptive transfer of wild-type and Il13(-/-) but not Il4(-/-) ILC2s restored sensitization in Il4raF709 Il1rl1(-/-) mice. Treg cells suppress ILC2s in vitro and in vivo.
Conclusion: IL-4 production by IL-33-stimulated ILC2s blocks the generation of allergen-specific Treg cells and favors food allergy. Strategies to block ILC2 activation or the IL-33/IL-33 receptor pathway can lead to innovative therapies in the treatment of food allergy.
Keywords: Anaphylaxis; IL-13; IL-33; IL-4; food allergy; group 2 innate lymphoid cells; innate lymphoid cells; mast cells; nuocytes; oral tolerance; regulatory T cells.
Copyright © 2016 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Figures


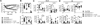

Comment in
-
Separating sensitization and regulatory T-cell functions in patients with food allergy.J Allergy Clin Immunol. 2016 Sep;138(3):812-813. doi: 10.1016/j.jaci.2016.06.007. Epub 2016 Jun 29. J Allergy Clin Immunol. 2016. PMID: 27464959 No abstract available.
Similar articles
-
IgE promotes type 2 innate lymphoid cells in murine food allergy.Clin Exp Allergy. 2018 Mar;48(3):288-296. doi: 10.1111/cea.13075. Epub 2018 Jan 22. Clin Exp Allergy. 2018. PMID: 29247574 Free PMC article.
-
Type 2 innate lymphoid cell suppression by regulatory T cells attenuates airway hyperreactivity and requires inducible T-cell costimulator-inducible T-cell costimulator ligand interaction.J Allergy Clin Immunol. 2017 May;139(5):1468-1477.e2. doi: 10.1016/j.jaci.2016.08.034. Epub 2016 Oct 4. J Allergy Clin Immunol. 2017. PMID: 27717665 Free PMC article.
-
A microbiota signature associated with experimental food allergy promotes allergic sensitization and anaphylaxis.J Allergy Clin Immunol. 2013 Jan;131(1):201-12. doi: 10.1016/j.jaci.2012.10.026. Epub 2012 Nov 30. J Allergy Clin Immunol. 2013. PMID: 23201093 Free PMC article.
-
IL-9-producing cells in the development of IgE-mediated food allergy.Semin Immunopathol. 2017 Jan;39(1):69-77. doi: 10.1007/s00281-016-0605-x. Epub 2016 Dec 1. Semin Immunopathol. 2017. PMID: 27909880 Free PMC article. Review.
-
Innate lymphoid cells in immunoglobulin E-mediated food allergy.Curr Opin Allergy Clin Immunol. 2024 Oct 1;24(5):419-425. doi: 10.1097/ACI.0000000000001018. Epub 2024 Aug 12. Curr Opin Allergy Clin Immunol. 2024. PMID: 39132724 Free PMC article. Review.
Cited by
-
PD-1+ CD4 T cell immune response is mediated by HIF-1α/NFATc1 pathway after P. yoelii infection.Front Immunol. 2022 Aug 24;13:942862. doi: 10.3389/fimmu.2022.942862. eCollection 2022. Front Immunol. 2022. PMID: 36091043 Free PMC article.
-
Oral Exposure to House Dust Mite Activates Intestinal Innate Immunity.Foods. 2021 Mar 9;10(3):561. doi: 10.3390/foods10030561. Foods. 2021. PMID: 33803079 Free PMC article.
-
ILCs and Allergy.Adv Exp Med Biol. 2022;1365:75-95. doi: 10.1007/978-981-16-8387-9_6. Adv Exp Med Biol. 2022. PMID: 35567742
-
Atopic Dermatitis Mediates the Association Between an IL4RA Variant and Food Allergy in School-Aged Children.J Allergy Clin Immunol Pract. 2022 Aug;10(8):2117-2124.e4. doi: 10.1016/j.jaip.2022.04.042. Epub 2022 May 16. J Allergy Clin Immunol Pract. 2022. PMID: 35589010 Free PMC article.
-
Early-life heterologous rhinovirus infections induce an exaggerated asthma-like phenotype.J Allergy Clin Immunol. 2020 Sep;146(3):571-582.e3. doi: 10.1016/j.jaci.2020.03.039. Epub 2020 Apr 25. J Allergy Clin Immunol. 2020. PMID: 32344055 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

