High-Fat Diet Promotion of Endometriosis in an Immunocompetent Mouse Model is Associated With Altered Peripheral and Ectopic Lesion Redox and Inflammatory Status
- PMID: 27175969
- PMCID: PMC4929556
- DOI: 10.1210/en.2016-1092
High-Fat Diet Promotion of Endometriosis in an Immunocompetent Mouse Model is Associated With Altered Peripheral and Ectopic Lesion Redox and Inflammatory Status
Abstract
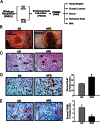
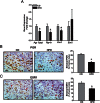
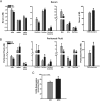
Endometriosis is a benign gynecological condition that causes considerable morbidity due to associated infertility, debilitating pelvic pain and inflammatory dysfunctions. Diet is a highly modifiable risk factor for many chronic diseases, but its contribution to endometriosis has not been extensively investigated, due partly to the paradoxical inverse association between obesity and disease incidence. Nevertheless, chronic exposure to dietary high-fat intake has been linked to greater systemic inflammation and oxidative stress, both features of women with endometriosis. Here, we evaluated the effects of a high-fat diet (HFD) (45% fat kcal) on endometriosis progression using an immunocompetent mouse model where ectopic lesion incidence was induced in wild-type recipients by ip administration of endometrial fragments from transcription factor Krüppel-like factor 9-null donor mice. We show that HFD significantly increased ectopic lesion numbers in recipient mice with no significant weight gain and modifications in systemic ovarian steroid hormone and insulin levels, relative to control diet-fed (17% fat kcal) mice. HFD promotion of lesion establishment was associated with reductions in stromal estrogen receptor 1 isoform and progesterone receptor expression, increased F4/80-positive macrophage infiltration, higher stromal but not glandular epithelial proliferation, and enhanced expression of proinflammatory and prooxidative stress pathway genes. Lesion-bearing HFD-fed mice also displayed higher peritoneal fluid TNFα and elevated local and systemic redox status than control diet-fed counterparts. Our results suggest that HFD intake exacerbates endometriosis outcome in the absence of ovarian dysfunction and insulin resistance in mice and warrants further consideration with respect to clinical management of endometriosis progression and recurrence in nonobese patients.
Figures


Similar articles
-
Lesion Genotype Modifies High-Fat Diet Effects on Endometriosis Development in Mice.Front Physiol. 2021 Sep 14;12:702674. doi: 10.3389/fphys.2021.702674. eCollection 2021. Front Physiol. 2021. PMID: 34712146 Free PMC article.
-
Krüppel-Like Factor 13 Deficiency in Uterine Endometrial Cells Contributes to Defective Steroid Hormone Receptor Signaling but Not Lesion Establishment in a Mouse Model of Endometriosis.Biol Reprod. 2015 Jun;92(6):140. doi: 10.1095/biolreprod.115.130260. Epub 2015 Apr 22. Biol Reprod. 2015. PMID: 25904015 Free PMC article.
-
Krüppel-like factor 9 deficiency in uterine endometrial cells promotes ectopic lesion establishment associated with activated notch and hedgehog signaling in a mouse model of endometriosis.Endocrinology. 2014 Apr;155(4):1532-46. doi: 10.1210/en.2013-1947. Epub 2014 Jan 29. Endocrinology. 2014. PMID: 24476135 Free PMC article.
-
Peritoneal endometriosis is an inflammatory disease.Front Biosci (Elite Ed). 2012 Jan 1;4(1):23-40. doi: 10.2741/e358. Front Biosci (Elite Ed). 2012. PMID: 22201853 Review.
-
The vicious cycle of chronic endometriosis and depression-an immunological and physiological perspective.Front Med (Lausanne). 2024 Sep 6;11:1425691. doi: 10.3389/fmed.2024.1425691. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39309679 Free PMC article. Review.
Cited by
-
The Molecular Link between Obesity and the Endometrial Environment: A Starting Point for Female Infertility.Int J Mol Sci. 2024 Jun 22;25(13):6855. doi: 10.3390/ijms25136855. Int J Mol Sci. 2024. PMID: 38999965 Free PMC article. Review.
-
Relationship Between Dairy Products Intake and Risk of Endometriosis: A Systematic Review and Dose-Response Meta-Analysis.Front Nutr. 2021 Jul 22;8:701860. doi: 10.3389/fnut.2021.701860. eCollection 2021. Front Nutr. 2021. PMID: 34368211 Free PMC article.
-
Western diet promotes endometriotic lesion growth in mice and induces depletion of Akkermansia muciniphila in intestinal microbiota.BMC Med. 2024 Nov 6;22(1):513. doi: 10.1186/s12916-024-03738-9. BMC Med. 2024. PMID: 39501247 Free PMC article.
-
High-fat diets promote peritoneal inflammation and augment endometriosis-associated abdominal hyperalgesia.bioRxiv [Preprint]. 2023 Nov 13:2023.11.09.566474. doi: 10.1101/2023.11.09.566474. bioRxiv. 2023. Update in: Front Endocrinol (Lausanne). 2024 Mar 15;15:1336496. doi: 10.3389/fendo.2024.1336496 PMID: 38014254 Free PMC article. Updated. Preprint.
-
Atherosclerosis and Endometriosis: The Role of Diet and Oxidative Stress in a Gender-Specific Disorder.Biomedicines. 2023 Feb 3;11(2):450. doi: 10.3390/biomedicines11020450. Biomedicines. 2023. PMID: 36830986 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

