Increases in Absolute Lymphocytes and Circulating CD4+ and CD8+ T Cells Are Associated with Positive Clinical Outcome of Melanoma Patients Treated with Ipilimumab
- PMID: 27169993
- PMCID: PMC5544386
- DOI: 10.1158/1078-0432.CCR-16-0249
Increases in Absolute Lymphocytes and Circulating CD4+ and CD8+ T Cells Are Associated with Positive Clinical Outcome of Melanoma Patients Treated with Ipilimumab
Abstract
Purpose: To investigate changes of peripheral blood biomarkers and their impact on clinical outcome following treatment with ipilimumab in advanced melanoma patients.
Experimental design: Changes in blood counts and the frequency of circulating immune cell populations analyzed by flow cytometry were investigated in 82 patients to compare baseline values with different time-points after starting ipilimumab. Endpoints were overall survival (OS) and best clinical response. Statistical calculations were done by Wilcoxon-matched pairs tests, Fisher exact test, Kaplan-Meier analysis, and Cox regression analysis.
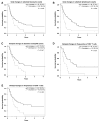
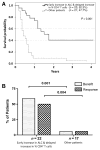
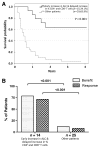
Results: Increases in absolute lymphocyte counts (ALC) 2 to 8 weeks (P = 0.003) and in percentages of CD4+ and CD8+ T cells 8 to 14 weeks (P = 0.001 and P = 0.02) after the first dose of ipilimumab were correlated with improved survival. These associations did not meet significance criteria, when conservatively adjusted for multiple testing, but were additionally correlated with clinical responses (all P < 0.05). However, validation is required. Increases in all three factors were observed in 36% of patients, who had a favorable outcome and survival probabilities of 93.3% and 63.8% at 12 and 24 months, respectively. A partial or complete response was observed in 71% of these patients compared with only 8% in patients with decreases in ≥1 of the 3 factors, respectively. Changes of regulatory T cells or myeloid-derived suppressor cells were not associated with OS.
Conclusions: Increases of ALC observed 2 to 8 weeks after initiation of ipilimumab and delayed increases in CD4+ and CD8+ T cells reflect changes associated with positive outcome. These changes represent surrogate marker candidates and warrant further validation. Clin Cancer Res; 22(19); 4848-58. ©2016 AACR.
©2016 American Association for Cancer Research.
Conflict of interest statement
J. Yuan is an employee of Merck. M.A. Postow reports receiving honoraria from Bristol-Myers Squibb and Merck, and is a consultant/advisory board member for and reports receiving commercial research grants from Bristol-Myers Squibb. P. Wong is a consultant/advisory board member for Merck. B. Schilling reports receiving speakers bureau honoraria from, is a consultant/advisory board member for, and reports receiving commercial research grants from Bristol-Myers Squibb. P.A. Ascierto is a consultant/advisory board member for Amgen, Array, Bristol-Myers Squibb, MSD, Novartis, Roche-Genentech, and Ventana, and reports receiving commercial research grants from Bristol-Myers Squibb, Roche-Genentech, and Ventana. J.D. Wolchok is a consultant/advisory board member for and reports receiving commercial research grants from Bristol-Myers Squibb and Medimmune. G. Pawelec reports receiving speakers bureau honoraria from Astellas, Cellgene, Clasado, and Pfizer. C. Garbe is a consultant/advisory board member for Amgen, Bristol-Myers Squibb, Leo Pharma, Merck/MSD, Novartis, Roche, and reports receiving commercial research grants from Bristol-Myers Squibb, Novartis, and Roche, and speakers bureau honoraria and travel reimbursement from Amgen, Bristol-Myers Squibb, Leo Pharma, Merck/MSD, Novartis, and Roche. B. Weide reports receiving commercial research grants, honoraria, and travel support from Bristol-Myers Squibb. No potential conflicts of interest were disclosed by the other authors.
Figures
Similar articles
-
Peripheral CD8 effector-memory type 1 T-cells correlate with outcome in ipilimumab-treated stage IV melanoma patients.Eur J Cancer. 2017 Mar;73:61-70. doi: 10.1016/j.ejca.2016.12.011. Epub 2017 Feb 4. Eur J Cancer. 2017. PMID: 28167454 Free PMC article.
-
Baseline Peripheral Blood Biomarkers Associated with Clinical Outcome of Advanced Melanoma Patients Treated with Ipilimumab.Clin Cancer Res. 2016 Jun 15;22(12):2908-18. doi: 10.1158/1078-0432.CCR-15-2412. Epub 2016 Jan 19. Clin Cancer Res. 2016. PMID: 26787752 Free PMC article.
-
Ipilimumab increases activated T cells and enhances humoral immunity in patients with advanced melanoma.J Immunother. 2012 Jan;35(1):89-97. doi: 10.1097/CJI.0b013e31823aa41c. J Immunother. 2012. PMID: 22130166 Clinical Trial.
-
Ipilimumab: a novel immunostimulatory monoclonal antibody for the treatment of cancer.Pharmacol Res. 2012 Jan;65(1):9-22. doi: 10.1016/j.phrs.2011.09.002. Epub 2011 Sep 10. Pharmacol Res. 2012. PMID: 21930211 Review.
-
Development of ipilimumab: contribution to a new paradigm for cancer immunotherapy.Semin Oncol. 2010 Oct;37(5):533-46. doi: 10.1053/j.seminoncol.2010.09.015. Semin Oncol. 2010. PMID: 21074069 Review.
Cited by
-
Clinical Pharmacokinetic and Pharmacodynamic Considerations in the (Modern) Treatment of Melanoma.Clin Pharmacokinet. 2019 Aug;58(8):1029-1043. doi: 10.1007/s40262-019-00753-5. Clin Pharmacokinet. 2019. PMID: 30868471 Review.
-
Prognostic biomarkers for immunotherapy with ipilimumab in metastatic melanoma.Clin Exp Immunol. 2019 Jul;197(1):74-82. doi: 10.1111/cei.13283. Epub 2019 Mar 21. Clin Exp Immunol. 2019. PMID: 30821848 Free PMC article. Clinical Trial.
-
Peripheral CD8 effector-memory type 1 T-cells correlate with outcome in ipilimumab-treated stage IV melanoma patients.Eur J Cancer. 2017 Mar;73:61-70. doi: 10.1016/j.ejca.2016.12.011. Epub 2017 Feb 4. Eur J Cancer. 2017. PMID: 28167454 Free PMC article.
-
Early decrease of blood myeloid-derived suppressor cells during checkpoint inhibition is a favorable biomarker in metastatic melanoma.J Immunother Cancer. 2023 Jun;11(6):e006802. doi: 10.1136/jitc-2023-006802. J Immunother Cancer. 2023. PMID: 37286306 Free PMC article.
-
Pretreatment antigen-specific immunity and regulation - association with subsequent immune response to anti-tumor DNA vaccination.J Immunother Cancer. 2017 Jul 18;5(1):56. doi: 10.1186/s40425-017-0260-3. J Immunother Cancer. 2017. PMID: 28716080 Free PMC article. Clinical Trial.
References
-
- Robert C, Thomas L, Bondarenko I, O’Day S, MDJ, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–26. - PubMed
-
- Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30–9. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

